Plasmid sequencing from DNA using SQK-RBK114 (.24 or .96) (PRB_9188_v114_revJ_27Oct2025)
MinION: Protocol
Plasmid sequencing from DNA using SQK-RBK114 (.24 or .96) V PRB_9188_v114_revJ_27Oct2025
This is the fastest and simplest protocol to sequence plasmid DNA.
The protocol:
- Is for multiplexing up to 96 samples
- Has a library preparation time of ~60 minutes
- Has a high yield
- Includes fragmentation
- Is compatible with R10.4.1 flow cells
For Research Use Only
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
Library preparation
Sequencing and data analysis
- 5. Data acquisition and basecalling
- 6. Downstream analysis using EPI2ME Labs
- 7. Flow cell reuse and returns
Troubleshooting
Overview
This is the fastest and simplest protocol to sequence plasmid DNA.
The protocol:
- Is for multiplexing up to 96 samples
- Has a library preparation time of ~60 minutes
- Has a high yield
- Includes fragmentation
- Is compatible with R10.4.1 flow cells
For Research Use Only
1. Overview of the protocol
Rapid Barcoding Kit features
This kit is recommended for users who:
- Wish to multiplex samples to reduce price per sample.
- Need a PCR-free method of multiplexing to preserve additional information such as base modifications.
- Require a short preparation time.
- Have limited access to laboratory equipment.
Introduction to plasmid sequencing using the Rapid Barcoding Kit 24 or 96 V14
This protocol describes how to carry out rapid barcoding of plasmid DNA using the Rapid Barcoding Kit 24 or 96 V14 (SQK-RBK114.24 or SQK-RBK114.96) to sequence up to 96 plasmid samples. This method can be utilised for routine verification of plasmid constructs in molecular biology research, quality control of plasmid DNA samples in biotechnology applications, and analysis of engineered plasmids for gene therapy development. During library preparation, the plasmid DNA is tagmented with the Rapid Barcodes before the samples are pooled and cleaned up. Rapid sequencing adapters are attached to the DNA ends before sequencing on a flow cell.
We recommend new users to sequence for 12 hours, although a shorter run-time may be sufficient. After sequencing, perform downstream analysis using the EPI2ME Labs Clone Validation (wf-clone-validation) workflow. A report is generated with a consensus sequence from each plasmid. Detailed instructions for setting up MinKNOW and the EPI2ME Labs workflow are included.
Steps in the sequencing workflow:
Prepare for your experiment
You will need to:
- Extract your DNA, and check its length, quantity and purity. The quality checks performed during the protocol are essential in ensuring experimental success.
- Ensure you have your sequencing kit, the correct equipment and third-party reagents.
- Download the software for acquiring and analysing your data.
- Check your flow cell to ensure it has enough pores for a good sequencing run.
Library preparation
You will need to:
- Tagment your DNA using the Rapid Barcodes; this simultaneously attaches a pair of barcodes to the fragments.
- Pool the barcoded samples.
- Attach the rapid sequencing adapters to the DNA ends.
- Prime the flow cell, and load your DNA library into the flow cell.

Sequencing and analysis
You will need to:
- Start a sequencing run using the MinKNOW software, which will collect raw data from the device into basecalled reads and will perform barcode demultiplexing.
- Start the EPI2ME software and use the Clone Validation workflow for analysis.
Compatibility of this protocol
This protocol should only be used in combination with:
- Rapid Barcoding Kit 24 V14 (SQK-RBK114.24)
- Rapid Barcoding Kit 96 V14 (SQK-RBK114.96)
- R10.4.1 flow cells (FLO-MIN114)
- Flow Cell Wash Kit (EXP-WSH004)
- Flow Cell Priming Kit V14 (EXP-FLP004)
- Sequencing Auxiliary Vials V14 (EXP-AUX003)
- Rapid Adapter Auxiliary V14 (EXP-RAA114)
- MinION Mk1D - MinION Mk1D IT requirements document
- GridION - GridION IT requirements document
2. Equipment and consumables
Materials
- 50 ng high molecular weight plasmid DNA per sample
- Rapid Barcoding Kit 24 V14 (SQK-RBK114.24) OR Rapid Barcoding Kit 96 V14 (SQK-RBK114.96)
Consumables
- 1.5 ml Eppendorf DNA LoBind tubes
- 2 ml Eppendorf DNA LoBind tubes
- 0.2 ml thin-walled PCR tubes or 0.2 ml 96-well PCR plate
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Freshly prepared 80% ethanol in nuclease-free water
- Bovine Serum Albumin (BSA) (50 mg/ml) (e.g Invitrogen™ UltraPure™ BSA 50 mg/ml, AM2616)
- Qubit™ Assay Tubes (Invitrogen, Q32856)
- Qubit dsDNA HS Assay Kit (Invitrogen, Q32851)
Equipment
- MinION or GridION device
- Ice bucket with ice
- Microplate centrifuge
- Timer
- Thermal cycler or heat blocks
- Magnetic separation rack
- Hula mixer (gentle rotator mixer)
- P1000 pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P2 pipette and tips
- Multichannel pipette and tips
- Qubit™ fluorometer (or equivalent for QC check)
Optional equipment
- Standard gel electrophoresis equipment
- Agilent Bioanalyzer (or equivalent)
For this protocol, you will need 50 ng high molecular weight plasmid DNA per sample.
Input DNA
How to QC your input DNA
It is important that the input DNA meets the quantity and quality requirements. Using too little or too much DNA, or DNA of poor quality (e.g. highly fragmented or containing RNA or chemical contaminants) can affect your library preparation.
For instructions on how to perform quality control of your DNA sample, please read the Input DNA/RNA QC protocol.
Chemical contaminants
Depending on how the DNA is extracted from the raw sample, certain chemical contaminants may remain in the purified DNA, which can affect library preparation efficiency and sequencing quality. Read more about contaminants on the Contaminants page of the Community.
Check your flow cell
We highly recommend that you check the number of pores in your flow cell prior to starting a sequencing experiment. This should be done within 12 weeks of purchasing your MinION/GridION Flow Cells. Oxford Nanopore Technologies will replace any unused flow cell with fewer than the number of pores listed in the Table below, when the result is reported within two days of performing the flow cell check, and when the storage recommendations have been followed. To do the flow cell check, please follow the instructions in the Flow Cell Check document.
| Flow cell | Minimum number of active pores covered by warranty |
|---|---|
| MinION/GridION Flow Cell | 800 |
The Rapid Adapter (RA) used in this kit and protocol is not interchangeable with other sequencing adapters.
Rapid Barcoding Kit 24 V14 (SQK-RBK114.24) contents
We are in the process of reformatting the barcodes provided in this kit into a plate format. This will reduce plastic waste and facilitates automated applications.
Plate format
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (µl) |
|---|---|---|---|---|
| Rapid Adapter | RA | Green | 1 | 15 |
| Adapter Buffer | ADB | Clear | 1 | 100 |
| AMPure XP Beads | AXP | Amber | 2 | 1200 |
| Elution Buffer | EB | Black | 1 | 500 |
| Sequencing Buffer | SB | Red | 1 | 700 |
| Library Beads | LIB | Pink | 1 | 600 |
| Library Solution | LIS | White cap, pink label | 1 | 600 |
| Flow Cell Flush | FCF | Clear cap, light blue label | 1 | 8000 |
| Flow Cell Tether | FCT | Purple | 1 | 200 |
| Rapid Barcode plate | RB01-24 | - | 2 plates, 3 sets of barcodes per plate | 5 µl per well |
This product contains AMPure XP reagent manufactured by Beckman Coulter, Inc. and can be stored at -20°C with the kit without detriment to reagent stability.
Vial format
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (µl) |
|---|---|---|---|---|
| Rapid Adapter | RA | Green | 1 | 15 |
| Adapter Buffer | ADB | Clear | 1 | 100 |
| AMPure XP Beads | AXP | Amber | 2 | 1,200 |
| Elution Buffer | EB | Black | 1 | 500 |
| Sequencing Buffer | SB | Red | 1 | 700 |
| Library Beads | LIB | Pink | 1 | 600 |
| Library Solution | LIS | White cap, pink label | 1 | 600 |
| Flow Cell Flush | FCF | Blue | 6 | 1,170 |
| Flow Cell Tether | FCT | Purple | 1 | 200 |
| Rapid Barcodes | RB01-24 | Clear | 24 | 15 |
This product contains AMPure XP reagent manufactured by Beckman Coulter, Inc. and can be stored at -20°C with the kit without detriment to reagent stability.
Rapid Barcoding Kit 96 V14 (SQK-RBK114.96) contents
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (µl) |
|---|---|---|---|---|
| Rapid Adapter | RA | Green | 2 | 15 |
| Adapter Buffer | ADB | Clear | 1 | 100 |
| AMPure XP Beads | AXP | Amber | 3 | 1,200 |
| Elution Buffer | EB | Black | 1 | 1,500 |
| Sequencing Buffer | SB | Red | 1 | 1,700 |
| Library Beads | LIB | Pink | 1 | 1,800 |
| Library Solution | LIS | White cap, pink label | 1 | 1,800 |
| Flow Cell Flush | FCF | Clear | 1 | 15,500 |
| Flow Cell Tether | FCT | Purple | 2 | 200 |
| Rapid Barcodes | RB01-96 | - | 3 plates | 8 µl per well |
This product contains AMPure XP reagent manufactured by Beckman Coulter, Inc. and can be stored at -20°C with the kit without detriment to reagent stability.
Rapid barcode sequences
| Component | Sequence |
|---|---|
| RB01 | AAGAAAGTTGTCGGTGTCTTTGTG |
| RB02 | TCGATTCCGTTTGTAGTCGTCTGT |
| RB03 | GAGTCTTGTGTCCCAGTTACCAGG |
| RB04 | TTCGGATTCTATCGTGTTTCCCTA |
| RB05 | CTTGTCCAGGGTTTGTGTAACCTT |
| RB06 | TTCTCGCAAAGGCAGAAAGTAGTC |
| RB07 | GTGTTACCGTGGGAATGAATCCTT |
| RB08 | TTCAGGGAACAAACCAAGTTACGT |
| RB09 | AACTAGGCACAGCGAGTCTTGGTT |
| RB10 | AAGCGTTGAAACCTTTGTCCTCTC |
| RB11 | GTTTCATCTATCGGAGGGAATGGA |
| RB12 | CAGGTAGAAAGAAGCAGAATCGGA |
| RB13 | AGAACGACTTCCATACTCGTGTGA |
| RB14 | AACGAGTCTCTTGGGACCCATAGA |
| RB15 | AGGTCTACCTCGCTAACACCACTG |
| RB16 | CGTCAACTGACAGTGGTTCGTACT |
| RB17 | ACCCTCCAGGAAAGTACCTCTGAT |
| RB18 | CCAAACCCAACAACCTAGATAGGC |
| RB19 | GTTCCTCGTGCAGTGTCAAGAGAT |
| RB20 | TTGCGTCCTGTTACGAGAACTCAT |
| RB21 | GAGCCTCTCATTGTCCGTTCTCTA |
| RB22 | ACCACTGCCATGTATCAAAGTACG |
| RB23 | CTTACTACCCAGTGAACCTCCTCG |
| RB24 | GCATAGTTCTGCATGATGGGTTAG |
| RB25 | GTAAGTTGGGTATGCAACGCAATG |
| RB26 | CATACAGCGACTACGCATTCTCAT |
| RB27 | CGACGGTTAGATTCACCTCTTACA |
| RB28 | TGAAACCTAAGAAGGCACCGTATC |
| RB29 | CTAGACACCTTGGGTTGACAGACC |
| RB30 | TCAGTGAGGATCTACTTCGACCCA |
| RB31 | TGCGTACAGCAATCAGTTACATTG |
| RB32 | CCAGTAGAAGTCCGACAACGTCAT |
| RB33 | CAGACTTGGTACGGTTGGGTAACT |
| RB34 | GGACGAAGAACTCAAGTCAAAGGC |
| RB35 | CTACTTACGAAGCTGAGGGACTGC |
| RB36 | ATGTCCCAGTTAGAGGAGGAAACA |
| RB37 | GCTTGCGATTGATGCTTAGTATCA |
| RB38 | ACCACAGGAGGACGATACAGAGAA |
| RB39 | CCACAGTGTCAACTAGAGCCTCTC |
| RB40 | TAGTTTGGATGACCAAGGATAGCC |
| RB41 | GGAGTTCGTCCAGAGAAGTACACG |
| RB42 | CTACGTGTAAGGCATACCTGCCAG |
| RB43 | CTTTCGTTGTTGACTCGACGGTAG |
| RB44 | AGTAGAAAGGGTTCCTTCCCACTC |
| RB45 | GATCCAACAGAGATGCCTTCAGTG |
| RB46 | GCTGTGTTCCACTTCATTCTCCTG |
| RB47 | GTGCAACTTTCCCACAGGTAGTTC |
| RB48 | CATCTGGAACGTGGTACACCTGTA |
| RB49 | ACTGGTGCAGCTTTGAACATCTAG |
| RB50 | ATGGACTTTGGTAACTTCCTGCGT |
| RB51 | GTTGAATGAGCCTACTGGGTCCTC |
| RB52 | TGAGAGACAAGATTGTTCGTGGAC |
| RB53 | AGATTCAGACCGTCTCATGCAAAG |
| RB54 | CAAGAGCTTTGACTAAGGAGCATG |
| RB55 | TGGAAGATGAGACCCTGATCTACG |
| RB56 | TCACTACTCAACAGGTGGCATGAA |
| RB57 | GCTAGGTCAATCTCCTTCGGAAGT |
| RB58 | CAGGTTACTCCTCCGTGAGTCTGA |
| RB59 | TCAATCAAGAAGGGAAAGCAAGGT |
| RB60 | CATGTTCAACCAAGGCTTCTATGG |
| RB61 | AGAGGGTACTATGTGCCTCAGCAC |
| RB62 | CACCCACACTTACTTCAGGACGTA |
| RB63 | TTCTGAAGTTCCTGGGTCTTGAAC |
| RB64 | GACAGACACCGTTCATCGACTTTC |
| RB65 | TTCTCAGTCTTCCTCCAGACAAGG |
| RB66 | CCGATCCTTGTGGCTTCTAACTTC |
| RB67 | GTTTGTCATACTCGTGTGCTCACC |
| RB68 | GAATCTAAGCAAACACGAAGGTGG |
| RB69 | TACAGTCCGAGCCTCATGTGATCT |
| RB70 | ACCGAGATCCTACGAATGGAGTGT |
| RB71 | CCTGGGAGCATCAGGTAGTAACAG |
| RB72 | TAGCTGACTGTCTTCCATACCGAC |
| RB73 | AAGAAACAGGATGACAGAACCCTC |
| RB74 | TACAAGCATCCCAACACTTCCACT |
| RB75 | GACCATTGTGATGAACCCTGTTGT |
| RB76 | ATGCTTGTTACATCAACCCTGGAC |
| RB77 | CGACCTGTTTCTCAGGGATACAAC |
| RB78 | AACAACCGAACCTTTGAATCAGAA |
| RB79 | TCTCGGAGATAGTTCTCACTGCTG |
| RB80 | CGGATGAACATAGGATAGCGATTC |
| RB81 | CCTCATCTTGTGAAGTTGTTTCGG |
| RB82 | ACGGTATGTCGAGTTCCAGGACTA |
| RB83 | TGGCTTGATCTAGGTAAGGTCGAA |
| RB84 | GTAGTGGACCTAGAACCTGTGCCA |
| RB85 | AACGGAGGAGTTAGTTGGATGATC |
| RB86 | AGGTGATCCCAACAAGCGTAAGTA |
| RB87 | TACATGCTCCTGTTGTTAGGGAGG |
| RB88 | TCTTCTACTACCGATCCGAAGCAG |
| RB89 | ACAGCATCAATGTTTGGCTAGTTG |
| RB90 | GATGTAGAGGGTACGGTTTGAGGC |
| RB91 | GGCTCCATAGGAACTCACGCTACT |
| RB92 | TTGTGAGTGGAAAGATACAGGACC |
| RB93 | AGTTTCCATCACTTCAGACTTGGG |
| RB94 | GATTGTCCTCAAACTGCCACCTAC |
| RB95 | CCTGTCTGGAAGAAGAATGGACTT |
| RB96 | CTGAACGGTCATAGAGTCCACCAT |
3. Library preparation
Materials
- 50 ng of high molecular weight plasmid DNA per sample
- Rapid Barcodes (RB01-24 or RB01-96)
- Rapid Adapter (RA)
- Adapter Buffer (ADB)
- AMPure XP Beads (AXP)
- Elution Buffer (EB)
Consumables
- 0.2 ml thin-walled PCR tubes or 0.2 ml 96-well PCR plate
- 1.5 ml Eppendorf DNA LoBind tubes
- 2 ml Eppendorf DNA LoBind tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Freshly prepared 80% ethanol in nuclease-free water
- Qubit™ Assay Tubes (Invitrogen, Q32856)
- Qubit dsDNA HS Assay Kit (Invitrogen, Q32851)
Equipment
- Ice bucket with ice
- Timer
- Thermal cycler
- Microplate centrifuge
- Magnetic separation rack
- Hula mixer (gentle rotator mixer)
- P1000 pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
- Multichannel pipette and tips
Program the thermal cycler: 30°C for 2 minutes, then 80°C for 2 minutes.
Check your flow cell.
We recommend performing a flow cell check before starting your library prep to ensure you have a flow cell with enough pores for a good sequencing run.
See the flow cell check document for more information.
Thaw kit components at room temperature, spin down briefly using a microfuge and mix by pipetting as indicated by the Table below:
| Reagent | 1. Thaw at room temperature | 2. Briefly spin down | 3. Mix well by pipetting |
|---|---|---|---|
| Rapid Barcodes (RB01-24 or RB01-96) | Not frozen | ✓ | ✓ |
| Rapid Adapter (RA) | Not frozen | ✓ | ✓ |
| AMPure XP Beads (AXP) | ✓ | ✓ | Mix by pipetting or vortexing immediately before use |
| Elution Buffer (EB) | ✓ | ✓ | ✓ |
| Adapter Buffer (ADB) | ✓ | ✓ | Mix by vortexing |
The wells of the barcoding plate are intended for single use only. Please ensure your barcode well is sealed before use, and do not reuse the barcode well once pierced/opened.
Prepare the DNA in nuclease-free water, as follows. Approximately 50 ng of plasmid DNA is required in 9 µl of volume for each sample for barcoding.
- Dilute your plasmid DNA samples with nuclease-free water to approximately 50 ng. See the table below for dilutions:
| Starting Conc. | Volume of DNA | Volume of nuclease-free water | Total volume |
|---|---|---|---|
| 100 ng/µl | 2 µl | 34 µl | 36 µl |
| 90 ng/µl | 2 µl | 31 µl | 33 µl |
| 80 ng/µl | 2 µl | 27 µl | 29 µl |
| 70 ng/µl | 3 µl | 35 µl | 38 µl |
| 60 ng/µl | 2 µl | 20 µl | 22 µl |
| 50 ng/µl | 2 µl | 16 µl | 18 µl |
| 40 ng/µl | 5 µl | 31 µl | 36 µl |
| 30 ng/µl | 5 µl | 22 µl | 27 µl |
| 20 ng/µl | 5 µl | 13 µl | 18 µl |
| 10 ng/µl | 10 µl | 8 µl | 18 µl |
| <5.56 ng/µl | 9 µl | 0 µl | 9 µl |
- Pipette mix the dilutions, and spin down briefly.
- Add 9 µl of volume for each sample into a 0.2 ml PCR tube or plate.
Select a unique barcode for every sample to be run together on the same flow cell. Up to 96 samples can be barcoded and combined in one experiment.
Please note: Only use one barcode per sample.
In 0.2 ml thin-walled PCR tubes or plate, mix the following reagents. The Rapid Barcodes can be transferred using a multichannel pipette:
| Reagent | Volume |
|---|---|
| 50 ng template DNA | 9 μl |
| Rapid Barcodes (RB01-96, one for each sample) | 1 μl |
| Total | 10 μl |
Ensure the components are thoroughly mixed by pipetting and spin down briefly.
Incubate the tubes or plate at 30°C for 2 minutes and then at 80°C for 2 minutes. Briefly put the tubes or plate on ice to cool.
Spin down the tubes or plate to collect the liquid at the bottom.
Pool all the barcoded samples into a clean 1.5 or 2 ml Eppendorf DNA LoBind tube, noting the total volume.
| . | Volume per sample | For 12 samples | For 24 samples | For 48 samples | For 96 samples |
|---|---|---|---|---|---|
| Total volume | 10 μl | 120 μl | 240 μl | 480 μl | 960 μl |
Resuspend the AMPure XP beads (AXP) by vortexing.
To the entire pooled barcoded sample, add an equal volume of resuspended AMPure XP Beads (AXP) and mix by flicking the tube.
| . | Volume per sample | For 12 samples | For 24 samples | For 48 samples | For 96 samples |
|---|---|---|---|---|---|
| Volume of AXP | 10 μl | 120 μl | 240 μl | 480 μl | 960 μl |
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Prepare at least 3 ml of fresh 80% ethanol in nuclease-free water.
Spin down the sample and pellet on a magnet. Keep the tube on the magnet, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 1.5 ml of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Briefly spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for 30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 15 µl Elution Buffer (EB). Incubate for 10 minutes at room temperature.
Pellet the beads on a magnet for at least 1 minute, until the eluate is clear and colourless.
Remove and retain 15 µl of eluate into a clean 1.5 ml Eppendorf DNA LoBind tube.
- Remove and retain the eluate which contains the DNA library in a clean 1.5 ml Eppendorf DNA LoBind tube.
- Dispose of the pelleted beads.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Transfer 11 µl of the sample into a clean 1.5 ml Eppendorf DNA LoBind tube.
Note: We recommend transfering a maximum of 800 ng of the DNA library. If necessary, take forward only the necessary volume for 800 ng of DNA library and make up the rest of the volume to 11 µl using Elution Buffer (EB).
In a fresh 1.5 ml Eppendorf DNA LoBind tube, dilute the Rapid Adapter (RA) as follows and pipette mix:
| Reagent | Volume |
|---|---|
| Rapid Adapter (RA) | 1.5 μl |
| Adapter Buffer (ADB) | 3.5 μl |
| Total | 5 μl |
Add 1 µl of the diluted Rapid Adapter (RA) to the barcoded DNA.
Mix gently by flicking the tube, and spin down.
Incubate the reaction for 5 minutes at room temperature.
The prepared library is used for loading into the flow cell. Store the library on ice until ready to load.
4. Priming and loading the MinION and GridION Flow Cell
Materials
- Flow Cell Flush (FCF)
- Flow Cell Tether (FCT)
- Library Solution (LIS)
- Library Beads (LIB)
- Sequencing Buffer (SB)
Consumables
- 1.5 ml Eppendorf DNA LoBind tubes
- MinION/GridION Flow Cell
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Bovine Serum Albumin (BSA) (50 mg/ml) (e.g Invitrogen™ UltraPure™ BSA 50 mg/ml, AM2616)
Equipment
- MinION or GridION device
- MinION/GridION Flow Cell Light Shield
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
Please note, this kit is only compatible with R10.4.1 flow cells (FLO-MIN114).
Take the flow cell out of the fridge and leave it at room temperature for 20 minutes. This will improve visibility of the array during priming and sample loading.
Priming and loading a flow cell
We recommend all new users watch the 'Priming and loading your flow cell' video before your first run.
Using the Library Solution
For most sequencing experiments, use the Library Beads (LIB) for loading your library onto the flow cell. However, for viscous libraries it may be difficult to load with the beads and may be appropriate to load using the Library Solution (LIS).
Thaw the Sequencing Buffer (SB), Library Beads (LIB) or Library Solution (LIS, if using), Flow Cell Tether (FCT) and Flow Cell Flush (FCF) at room temperature before mixing by vortexing. Then spin down and store on ice.
For optimal sequencing performance and improved output on MinION R10.4.1 flow cells (FLO-MIN114), add Bovine Serum Albumin (BSA) to the flow cell priming mix at a final concentration of 0.2 mg/ml.
Note: We do not recommend using any other albumin type (e.g. recombinant human serum albumin).
To prepare the flow cell priming mix with BSA, combine the following reagents in a suitable tube and pipette mix at room temperature:
| Reagents | Volume per flow cell |
|---|---|
| Flow Cell Flush (FCF) | 1,170 µl |
| Bovine Serum Albumin (BSA) at 50 mg/ml | 5 µl |
| Flow Cell Tether (FCT) | 30 µl |
| Total volume | 1,205 µl |
Open the MinION or GridION device lid and slide the flow cell under the clip. Press down firmly on the priming port cover to ensure correct thermal and electrical contact.
Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check document for more information.
Slide the flow cell priming port cover clockwise to open the priming port.
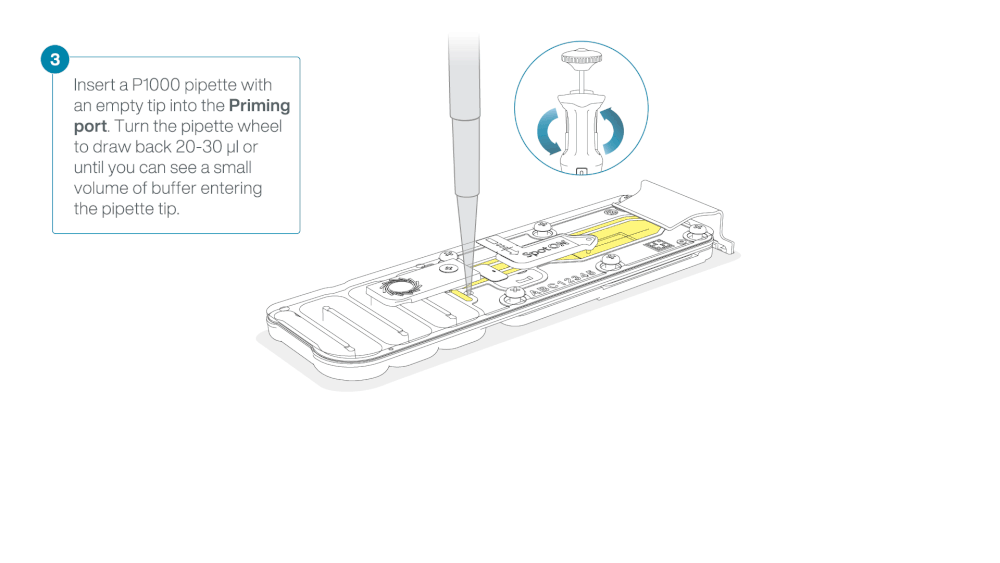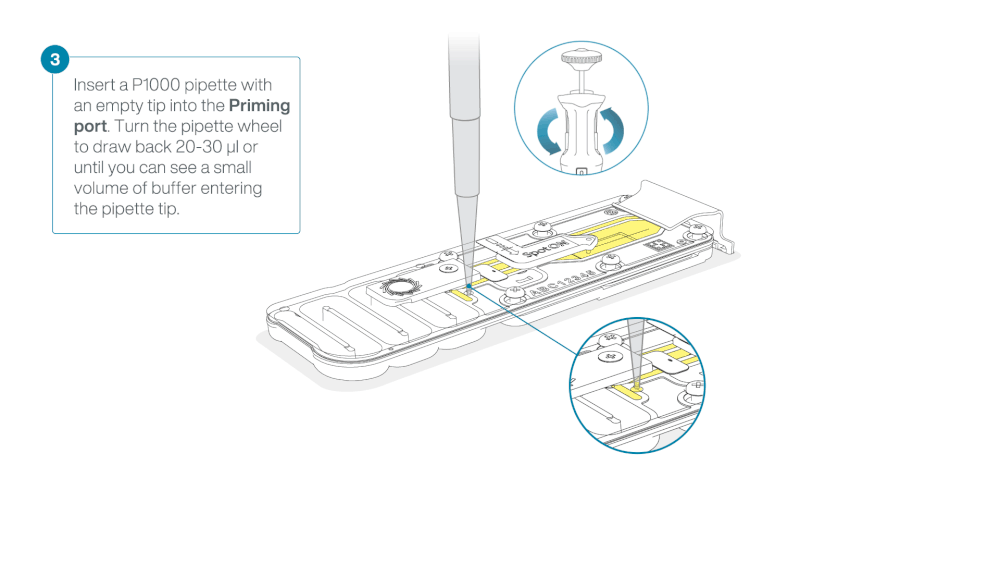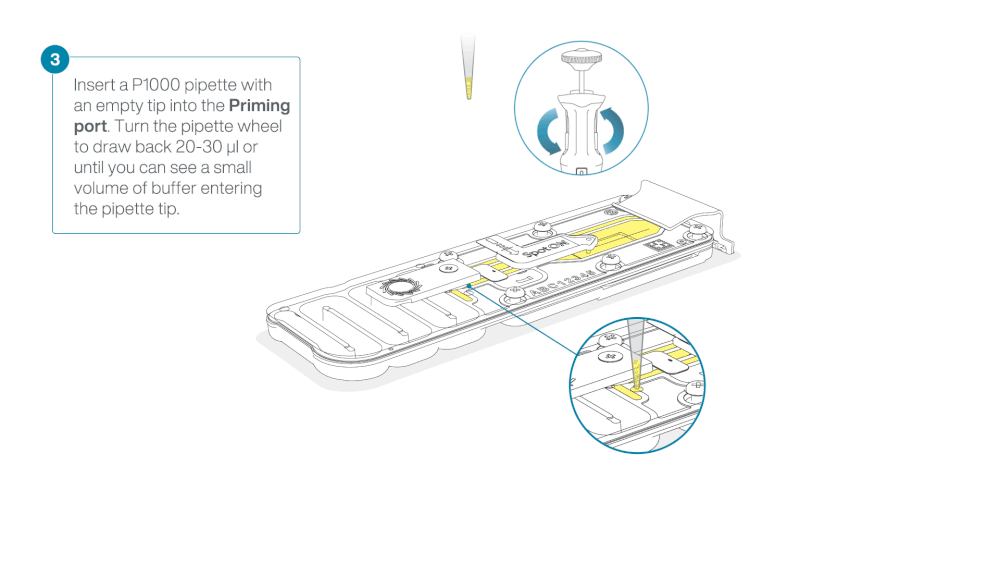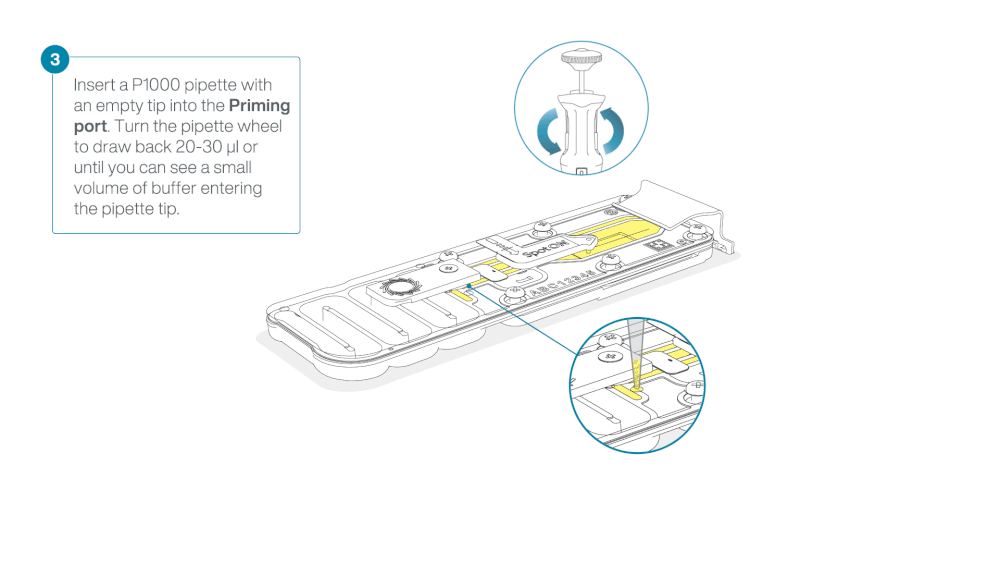
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 µl, to draw back 20-30 µl, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the Library Beads (LIB) by pipetting.
The Library Beads (LIB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
We recommend using the Library Beads (LIB) for most sequencing experiments. However, the Library Solution (LIS) is available for more viscous libraries.
In a new 1.5 ml Eppendorf DNA LoBind tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SB) | 37.5 µl |
| Library Beads (LIB) mixed immediately before use, or Library Solution (LIS), if using | 25.5 µl |
| DNA library | 12 µl |
| Total | 75 µl |
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port and close the priming port.

For optimal sequencing output, install the light shield on your flow cell as soon as the library has been loaded.
We recommend leaving the light shield on the flow cell when library is loaded, including during any washing and reloading steps. The shield can be removed when the library has been removed from the flow cell.
Place the light shield onto the flow cell, as follows:
Carefully place the leading edge of the light shield against the clip. Note: Do not force the light shield underneath the clip.
Gently lower the light shield onto the flow cell. The light shield should sit around the SpotON cover, covering the entire top section of the flow cell.

The MinION Flow Cell Light Shield is not secured to the flow cell and careful handling is required after installation.
Close the device lid and set up a sequencing run on MinKNOW.
When a flow cell is inserted into the MinION Mk1D, the device lid will sit on top of the flow cell, leaving a small gap around the sides. This is normal and has no impact on the performance of the device.
Please refer to this FAQ regarding the device lid.

5. Data acquisition and basecalling
How to start sequencing
The sequencing device control, data acquisition and real-time basecalling are carried out by the MinKNOW software. Please ensure MinKNOW is installed on your computer or device. Further instructions for setting up a sequencing run can be found in the MinKNOW protocol.
We recommend setting up a sequencing run on a MinION or GridION device using the basecalling and barcoding recommendations outlined below. All other parameters can be left to their default settings.
For more information on using MinKNOW on a sequencing device, please see the device user manuals:
Open the MinKNOW software using the desktop shortcut and log into the MinKNOW software using your Community credentials.
Click on your connected device.
Set up a sequencing run by clicking Start sequencing.
Type in the experiment name, select the flow cell postition and enter sample ID. Choose FLO-MIN114 flow cell type from the drop-down menu.
Click Continue to kit selection.
Select the Rapid Barcoding Kit 24 (SQK-RBK114-24) or Rapid Barcoding Kit 96 (SQK-RBK114-96).
Click Continue to Run Options.
Change the run limit to 12 hours by clicking "Options" and changing the run limit value to 12. The other run settings can be left as default.
Click Continue to basecalling.
Set up basecalling and barcoding using the following parameters:
Ensure basecalling is ON.
Next to "Models", click Edit options and choose High accuracy basecaller (HAC) from the drop-down menu.
Ensure barcoding in ON.
All other options can be kept to their default settings.
Click Continue to output.
Set up the output format and filtering as follows:
Select either .POD5 or .FAST5 (legacy) as the output format.
Ensure .FASTQ is selected for basecalled reads.
Ensure filtering is ON and read splitting is enabled. Other parameters can be kept to their default settings.
Click Continue to final review.
Click "Start" to start sequencing.
You will be automatically navigated to the "Sequencing Overview" page to monitor the sequencing run.
6. Downstream analysis using EPI2ME Labs
Post-basecalling analysis
We recommend performing downstream analysis using EPI2ME Labs which facilitates bioinformatic analyses by allowing users to run Nextflow workflows in a desktop application. EPI2ME Labs maintains a collection of bioinformatic workflows which are curated and actively maintained by experts in long-read sequence analysis.
Further information about the available EPI2ME Labs workflows are available here, along with the Quick Start Guide to start your first bioinformatic workflow.
For the assembly of small plasmid sequences, we recommend using the wf-clone-validation workflow which requires Nextflow and Docker to be installed before running the workflow.
Open the EPI2ME app using the desktop shortcut.
Scroll down on the landing page and click on the wf-clone-validation workflow to download and confirm to install.


Navigate to the Workflows tab and click on wf-clone-validation.

Click on "Run this workflow" to open the launch wizard.

Set up your run by uploading your FASTQ file in the "Input Options". We recommend keeping the default settings for the other parameter options.

Click "Launch workflow".
Ensure all parameter options have green ticks.

Once the workflow finishes, a report will be produced.
Clone validation workflow report
A report is produced containing the results of the assembled plasmid sequences. The primary outputs of the workflow include:
- A consensus FASTA file for each sample.
- A CSV showing the pass or fail status of each sample.
- A feature Table containing annotations for each of the samples.
- An HTML report document detailing the primary findings of the workflow.
Please refer to the sample report.
 The summary graph shows the number of reads per barcode and the table shows the length of the consensus sequence for each barcode. These data may be used to identify the samples that may have dropped out of the sequence analysis due to insufficient sequence reads.
The summary graph shows the number of reads per barcode and the table shows the length of the consensus sequence for each barcode. These data may be used to identify the samples that may have dropped out of the sequence analysis due to insufficient sequence reads.
 For each barcode, read length statistics and a pLannotate plot is presented to illustrate the polished plasmid consensus sequence. In the sample report, barcode01 has been assembled into a 5385 bp consensus sequence. The unfilled features on the plot are incomplete features.
For each barcode, read length statistics and a pLannotate plot is presented to illustrate the polished plasmid consensus sequence. In the sample report, barcode01 has been assembled into a 5385 bp consensus sequence. The unfilled features on the plot are incomplete features.
 A feature Table is also provided for each barcode to give descriptions of the annotated sequence which can be used to identify the precise location of the annotated features.
A feature Table is also provided for each barcode to give descriptions of the annotated sequence which can be used to identify the precise location of the annotated features.
7. Flow cell reuse and returns
Materials
- Flow Cell Wash Kit (EXP-WSH004)
After your sequencing experiment is complete, if you would like to reuse the flow cell, please follow the Flow Cell Wash Kit protocol and store the washed flow cell at +2°C to +8°C.
The Flow Cell Wash Kit protocol is available on the Nanopore Community.
Alternatively, follow the returns procedure to send the flow cell back to Oxford Nanopore.
Instructions for returning flow cells can be found here.
If you encounter issues or have questions about your sequencing experiment, please refer to the Troubleshooting Guide that can be found in this protocol.
8. Issues during DNA extraction and library preparation
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Low sample quality
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low DNA purity (Nanodrop reading for DNA OD 260/280 is <1.8 and OD 260/230 is <2.0–2.2) | The DNA extraction method does not provide the required purity | The effects of contaminants are shown in the Contaminants document. Please try an alternative extraction method that does not result in contaminant carryover. Consider performing an additional SPRI clean-up step. |
Low DNA recovery after AMPure bead clean-up
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low recovery | DNA loss due to a lower than intended AMPure beads-to-sample ratio | 1. AMPure beads settle quickly, so ensure they are well resuspended before adding them to the sample. 2. When the AMPure beads-to-sample ratio is lower than 0.4:1, DNA fragments of any size will be lost during the clean-up. |
| Low recovery | DNA fragments are shorter than expected | The lower the AMPure beads-to-sample ratio, the more stringent the selection against short fragments. Please always determine the input DNA length on an agarose gel (or other gel electrophoresis methods) and then calculate the appropriate amount of AMPure beads to use. 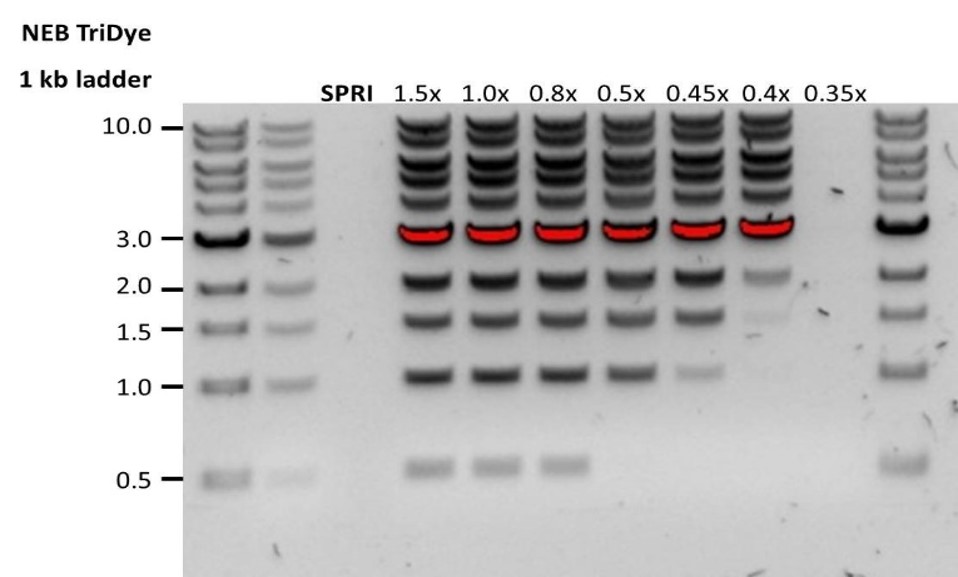 |
| Low recovery after end-prep | The wash step used ethanol <70% | DNA will be eluted from the beads when using ethanol <70%. Make sure to use the correct percentage. |
9. Issues during the sequencing run
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Fewer pores at the start of sequencing than after Flow Cell Check
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | An air bubble was introduced into the nanopore array | After the Flow Cell Check it is essential to remove any air bubbles near the priming port before priming the flow cell. If not removed, the air bubble can travel to the nanopore array and irreversibly damage the nanopores that have been exposed to air. The best practice to prevent this from happening is demonstrated in this video. |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | The flow cell is not correctly inserted into the device | Stop the sequencing run, remove the flow cell from the sequencing device and insert it again, checking that the flow cell is firmly seated in the device and that it has reached the target temperature. If applicable, try a different position on the device (GridION/PromethION). |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | Contaminations in the library damaged or blocked the pores | The pore count during the Flow Cell Check is performed using the QC DNA molecules present in the flow cell storage buffer. At the start of sequencing, the library itself is used to estimate the number of active pores. Because of this, variability of about 10% in the number of pores is expected. A significantly lower pore count reported at the start of sequencing can be due to contaminants in the library that have damaged the membranes or blocked the pores. Alternative DNA/RNA extraction or purification methods may be needed to improve the purity of the input material. The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
MinKNOW script failed
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Script failed" | Restart the computer and then restart MinKNOW. If the issue persists, please collect the MinKNOW log files and contact Technical Support. If you do not have another sequencing device available, we recommend storing the flow cell and the loaded library at 4°C and contact Technical Support for further storage guidance. |
Pore occupancy below 40%
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Pore occupancy <40% | Not enough library was loaded on the flow cell | Ensure you load the recommended amount of good quality library in the relevant library prep protocol onto your flow cell. Please quantify the library before loading and calculate mols using tools like the Promega Biomath Calculator, choosing "dsDNA: µg to pmol" |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and sequencing adapters did not ligate to the DNA | Make sure to use the NEBNext Quick Ligation Module (E6056) and Oxford Nanopore Technologies Ligation Buffer (LNB, provided in the sequencing kit) at the sequencing adapter ligation step, and use the correct amount of each reagent. A Lambda control library can be prepared to test the integrity of the third-party reagents. |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and ethanol was used instead of LFB or SFB at the wash step after sequencing adapter ligation | Ethanol can denature the motor protein on the sequencing adapters. Make sure the LFB or SFB buffer was used after ligation of sequencing adapters. |
| Pore occupancy close to 0 | No tether on the flow cell | Tethers are adding during flow cell priming (FLT/FCT tube). Make sure FLT/FCT was added to FB/FCF before priming. |
Shorter than expected read length
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Shorter than expected read length | Unwanted fragmentation of DNA sample | Read length reflects input DNA fragment length. Input DNA can be fragmented during extraction and library prep. 1. Please review the Extraction Methods in the Nanopore Community for best practice for extraction. 2. Visualise the input DNA fragment length distribution on an agarose gel before proceeding to the library prep. 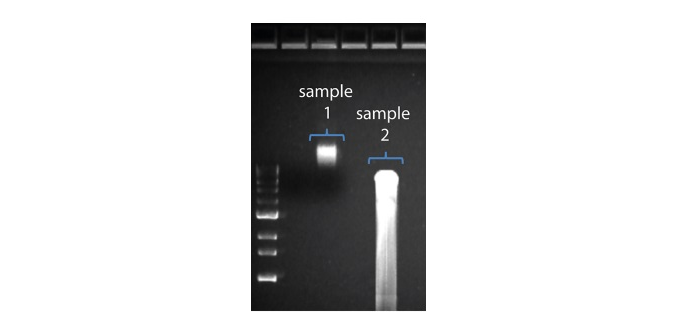 In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented. In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented.3. During library prep, avoid pipetting and vortexing when mixing reagents. Flicking or inverting the tube is sufficient. |
Large proportion of unavailable pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
Large proportion of unavailable pores (shown as blue in the channels panel and pore activity plot) 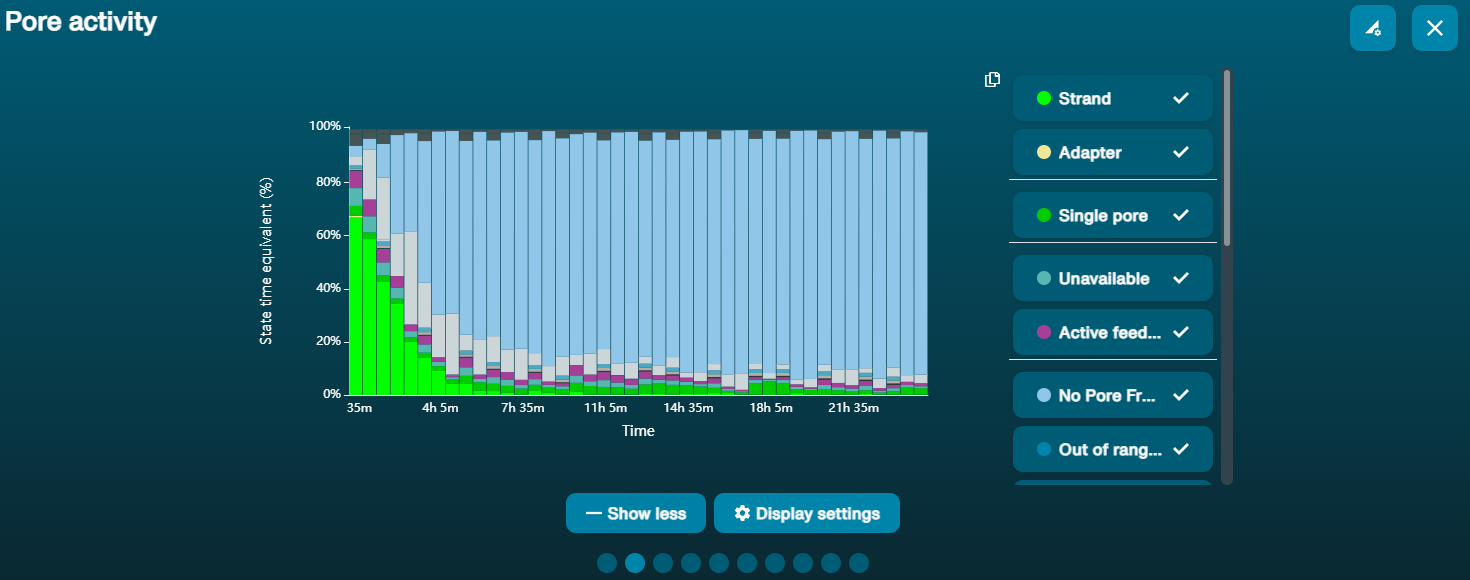 The pore activity plot above shows an increasing proportion of "unavailable" pores over time. The pore activity plot above shows an increasing proportion of "unavailable" pores over time. | Contaminants are present in the sample | Some contaminants can be cleared from the pores by the unblocking function built into MinKNOW. If this is successful, the pore status will change to "sequencing pore". If the portion of unavailable pores stays large or increases: 1. A nuclease flush using the Flow Cell Wash Kit (EXP-WSH004) can be performed, or 2. Run several cycles of PCR to try and dilute any contaminants that may be causing problems. |
Large proportion of inactive pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Large proportion of inactive/unavailable pores (shown as light blue in the channels panel and pore activity plot. Pores or membranes are irreversibly damaged) | Air bubbles have been introduced into the flow cell | Air bubbles introduced through flow cell priming and library loading can irreversibly damage the pores. Watch the Priming and loading your flow cell video for best practice. |
| Large proportion of inactive/unavailable pores | Certain compounds co-purified with DNA | Known compounds include polysaccharides. Clean-up using the QIAGEN PowerClean Pro kit. |
| Large proportion of inactive/unavailable pores | Contaminants are present in the sample | The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
Temperature fluctuation
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Temperature fluctuation | The flow cell has lost contact with the device | Check that there is a heat pad covering the metal plate on the back of the flow cell. Re-insert the flow cell and press it down to make sure the connector pins are firmly in contact with the device. If the problem persists, please contact Technical Services. |
Failed to reach target temperature
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Failed to reach target temperature" | The instrument was placed in a location that is colder than normal room temperature, or a location with poor ventilation (which leads to the flow cells overheating) | MinKNOW has a default timeframe for the flow cell to reach the target temperature. Once the timeframe is exceeded, an error message will appear and the sequencing experiment will continue. However, sequencing at an incorrect temperature may lead to a decrease in throughput and lower q-scores. Please adjust the location of the sequencing device to ensure that it is placed at room temperature with good ventilation, then re-start the process in MinKNOW. Please refer to this link for more information on MinION temperature control. |







