PCR tiling of SARS-CoV-2 virus - classic protocol (SQK-LSK109 with EXP-NBD104, EXP-NBD114 or EXP-NBD196) (PTC_9096_v109_revY_06Feb2020)
MinION: Protocol
PCR tiling of SARS-CoV-2 virus - classic protocol (SQK-LSK109 with EXP-NBD104, EXP-NBD114 or EXP-NBD196) V PTC_9096_v109_revY_06Feb2020
For Research Use Only This protocol includes a PCR SPRI clean-up, quantification and normalisation steps to ensure equal distribution of barcodes for 24, 48 and 96 samples.
This is a Legacy product This kit is available on the Legacy page of the store. We are in the process of gathering data to support the upgrade of this protocol to our latest chemistry. Further information regarding protocol upgrades will be provided on the Community as soon as they are available over the next few months.
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
Library preparation
- 4. Reverse transcription
- 5. PCR and clean-up
- 6. End-prep
- 7. Native barcode ligation
- 8. Adapter ligation and clean-up
- 9. Priming and loading the SpotON flow cell
Sequencing and data analysis
- 10. Data acquisition and basecalling
- 11. Downstream analysis and expected results
- 12. Flow cell reuse and returns
Troubleshooting
Overview
For Research Use Only This protocol includes a PCR SPRI clean-up, quantification and normalisation steps to ensure equal distribution of barcodes for 24, 48 and 96 samples.
This is a Legacy product This kit is available on the Legacy page of the store. We are in the process of gathering data to support the upgrade of this protocol to our latest chemistry. Further information regarding protocol upgrades will be provided on the Community as soon as they are available over the next few months.
1. Overview of the protocol
This is a Legacy product
This kit is available on the Legacy page of the store. We are in the process of gathering data to support the upgrade of this protocol to our latest chemistry. Further information regarding protocol upgrades will be provided on the Community as soon as they are available over the next few months. For further information on please see the product update page.
This protocol is a work in progress, and some details are expected to change over time. Please make sure you always use the most recent version of the protocol.
This protocol is the 'classic' version of the PCR tiling of SARS-CoV-2 virus protocol, which includes a PCR SPRI clean-up, quantification and normalisation steps to ensure equal distribution of barcodes. This protocol has been updated to outline library preparation for X24, X48 and X96 samples using the Native Barcoding Expansions 1-12 (EXP-NBD104), 13-24 (EXP-NBD114), or Native Barcoding Expansion 96 (EXP-NBD196).
This protocol is based on the ARTIC amplicon sequencing protocol for MinION for SARS-CoV-2 v3 (LoCost) by Josh Quick. In the table below, we have highlighted which steps are different between the protocols.
| Step change | Oxford Nanopore Technologies protocol PCR tiling of SARS-CoV-2 virus | Oxford Nanopore Technologies protocol Eco PCR tiling of SARS-CoV-2 virus | ARTIC amplicon sequencing protocol for SARS-CoV-2 v3 (LoCost) by Josh Quick |
|---|---|---|---|
| Reverse transcription | LunaScript RT SuperMix (5X): 4 µl RNA sample: 16 µl Total: 20 µl | LunaScript RT SuperMix (5X): 2 µl RNA sample: 8 µl Total: 10 µl | LunaScript RT SuperMix (5X): 2 µl RNA sample: 8 µl Total: 10 µl |
| PCR | Q5 Hot Start High-Fidelity 2X Master Mix: 12.5 µl Primer pool A/B (10 µM): 3.7 µl Nuclease-free water: 3.8 µl Total: 20 µl cDNA: 5 µl 1.0X SPRI clean-up after PCR | Q5 Hot Start High-Fidelity 2X Master Mix: 12.5 µl Primer pool A/B (100 µM): 0.37 µl Nuclease-free water: 9.63 µl Total: 22.5 µl cDNA: 2.5 µl The clean-up, quantification and normalisation steps have been removed. | Q5 Hot Start High-Fidelity 2X Master Mix: 12.5 µl Primer pool A/B (10 µM): 4 µl Nuclease-free water: 6 µl Total: 22.5 µl cDNA: 2.5 µl |
| End-prep | DNA in nuclease-free water: 12.5 µl Ultra II End Prep Reaction Buffer: 1.75 µl Ultra II End Prep Enzyme Mix: 0.75 µl Total: 15 µl | DNA: 3.3 µl Nuclease-free water: 5 µl Ultra II End Prep Reaction Buffer: 1.2 µl Ultra II End Prep Enzyme Mix: 0.5 µl Total: 10 µl | DNA: 3.3 µl Nuclease-free water: 5 µl Ultra II End Prep Reaction Buffer: 1.2 µl Ultra II End Prep Enzyme Mix: 0.5 µl Total: 10 µl |
| Native barcode ligation x24 | Nuclease-free water: 6 µl DNA 1.5 µl µl Native barcode: 2.5 µl Blunt/TA Ligase Master Mix: 10 µl Total: 20 µl | Nuclease-free water: 6 µl DNA 1.5 µl µl Native barcode: 2.5 µl Blunt/TA Ligase Master Mix: 10 µl Total: 20 µl | Nuclease-free water: 3 µl DNA 0.75 µl Native barcode: 1.25 µl Blunt/TA Ligase Master Mix: 5 µl Total: 10 µl |
| Native barcode ligation for x48 and x96 | Nuclease-free water: 3 µl DNA: 0.75 µl Native barcode: 1.25 µl Blunt/TA Ligase Master Mix: 5 µl Total: 10 µl | Nuclease-free water: 3 µl DNA: 0.75 µl Native barcode: 1.25 µl Blunt/TA Ligase Master Mix: 5 µl Total: 10 µl | Same as Native barcode ligation for x24 (above) |
| Pooled barcoded samples | 480 µl | 480 µl | Maximum 240 µl |
| Native barcode ligation clean-up | SFB: 700 µl 80% ethanol wash | SFB: 700 µl 80% ethanol wash | SFB: 250 µl 70% ethanol wash |
| Adapter ligation clean-up | 0.4X SPRI bead clean-up SFB: 125 µl | 0.4X SPRI bead clean-up SFB: 125 µl | 1.0X SPRI bead clean-up SFB: 250 µl |
Introduction to the protocol
To enable the support for the rapidly expanding user requests, the team at Oxford Nanopore Technologies have put together an end-to-end workflow based on the ARTIC Network protocols and analysis methods.
While this protocol is available in the Nanopore Community, we kindly ask users to ensure they are citing the members of the ARTIC network who have been behind the development of these methods.
This protocol is based on the ARTIC amplicon sequencing protocol for MinION for nCOV-2019 by Josh Quick. The protocol generates 400 bp amplicons in a tiled fashion across the whole SARS-CoV-2 genome. Some example data is shown in the Downstream analysis and expected results section, this is generated using human coronavirus 229E to show what would be expected when running this protocol with SARS-CoV-2 samples.
Primers were designed by Josh Quick using Primal Scheme; the primer sequences can be found here.
Steps in the sequencing workflow:
Prepare for your experiment you will need to:
- Extract your RNA
- Ensure you have your sequencing kit, the correct equipment and third-party reagents
- Download the software for acquiring and analysing your data
- Check your flow cell to ensure it has enough pores for a good sequencing run
Prepare your library You will need to:
- Reverse transcribe your RNA samples with random hexamers
- Amplify the samples by tiled PCR using separate primer pools
- Combine the primer pools, purify and quantify the PCR products
- Prepare the DNA ends for adapter attachment
- Ligate native barcodes supplied in the kit to the DNA ends and pool the samples
- Ligate the sequencing adapters supplied in the kit to the DNA ends
- Prime the flow cell and load your DNA library into the flow cell
Sequencing and analysis You will need to:
- Start a sequencing run using the MinKNOW software, which will collect raw data from the device and convert it into basecalled reads
- Start the EPI2ME software and select the barcoding workflow
Before starting
This protocol requires total RNA extracted from samples that have been screened by a suitable qPCR assay. Here we demonstrate the level of sensitivity and specificity by titrating total RNA extracted from cell culture infected with Human coronavirus 229E spiked into 100 ng human RNA extracted from GM12878 to give approximate figures.
Although not tested here, work performed by Josh Quick et al. on the Zika virus gives approximate dilution factors that may help reduction of inhibiting compounds that can be co-extracted from samples.
Note: this is a guideline and not currently tested for SARS-CoV-2.
| qPCR ct | Dilution factor |
|---|---|
| 18–35 | none |
| 15–18 | 1:10 |
| 12–15 | 1:100 |
When processing multiple samples at once, we recommend making master mixes with an additional 10% of the volume. We also recommend using pre- and post-PCR hoods when handling master mixes and samples. It is important to clean and/or UV irradiate these hoods between sample batches. Furthermore, to track and monitor cross-contamination events, it is important to run a negative control reaction at the reverse transcription stage using nuclease-free water instead of sample, and carrying this control through the rest of the prep.
To minimise the chance of pipetting errors when preparing primer mixes, we recommend ordering the tiling primers from IDT in a lab-ready format at 100 µM.
Compatibility of this protocol
This protocol should only be used in combination with:
- Native Barcoding Expansion 1-12 (EXP-NBD104) and 13-24 (EXP-NBD114)
- Native Barcoding Expansion 96 (EXP-NBD196)
- Ligation Sequencing Kit (SQK-LSK109)
- FLO-MIN106 (R9.4.1) flow cells
- Flow Cell Wash Kit (EXP-WSH004)
- SFB Expantion (EXP-SFB001)
- Sequencing Auxiliary Kit (EXP-AUX001)
2. Equipment and consumables
Materials
- Input RNA in 10 mM Tris-HCl, pH 8.0
- Ligation Sequencing Kit (SQK-LSK109)
- Native Barcoding Expansion 1-12 (EXP-NBD104) or 13-24 (EXP-NBD114)
- Native Barcoding Expansion 96 (EXP-NBD196)
- Flow Cell Priming Kit (EXP-FLP002)
- SFB Expansion (EXP-SFB001)
- Adapter Mix II Expansion (EXP-AMII001)
Consumables
- LunaScript™ RT SuperMix Kit (NEB, cat # E3010)
- Q5® Hot Start High-Fidelity 2X Master Mix (NEB, cat # M0494)
- SARS-CoV-2 primers (lab-ready at 100 µM, IDT)
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Agencourt AMPure XP beads (Beckman Coulter™, A63881)
- Freshly prepared 80% ethanol in nuclease-free water
- Qubit™ dsDNA HS Assay Kit (ThermoFisher, Q32851)
- NEB Blunt/TA Ligase Master Mix (NEB, M0367)
- NEBNext® Ultra™ II End Repair/dA-Tailing Module (NEB, E7546)
- NEBNext Quick Ligation Module (NEB, E6056)
- DNA 12000 Kit & Reagents - optional (Agilent Technologies)
- 1.5 ml Eppendorf DNA LoBind tubes
- Eppendorf twin.tec® PCR plate 96 LoBind, semi-skirted (Merck, EP0030129504) with heat seals
Equipment
- Hula mixer (gentle rotator mixer)
- Magnetic rack suitable for 96-well PCR plates, e.g. DynaMag™-96 Side Skirted Magnet (Thermo Fisher,12027)
- Microplate centrifuge
- Vortex mixer
- Thermal cycler
- Stepper pipette and tips
- Multichannel pipette and tips
- P1000 pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
- Ice bucket with ice
- Timer
Optional equipment
- Agilent Bioanalyzer (or equivalent)
- Qubit™ fluorometer (or equivalent for QC check)
- Eppendorf 5424 centrifuge (or equivalent)
- PCR hood with UV steriliser (optional but recommended to reduce cross-contamination)
- PCR-Cooler (Eppendorf)
Input RNA guidelines
Where sample RNA is added to the below reaction, it is likely advantageous to follow the dilution guidelines proposed by Josh Quick:
| qPCR Ct | Dilution factor |
|---|---|
| 18–35 | none |
| 15–18 | 1:10 |
| 12–15 | 1:100 |
If the sample has a low copy number (ct 18–35) use up to 16 µl of sample. Use nuclease-free water to make up any remaining volume. Take note to be aware that co-extracted compounds may inhibit reverse transcription and PCR.
Ligation Sequencing Kit contents (SQK-LSK109)
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (µl) |
|---|---|---|---|---|
| DNA CS | DCS | Yellow | 1 | 50 |
| Adapter Mix | AMX | Green | 1 | 40 |
| Ligation Buffer | LNB | Clear | 1 | 200 |
| L Fragment Buffer | LFB | White cap, orange stripe on label | 2 | 1,800 |
| S Fragment Buffer | SFB | Grey | 2 | 1,800 |
| Sequencing Buffer | SQB | Red | 2 | 300 |
| Elution Buffer | EB | Black | 1 | 200 |
| Loading Beads | LB | Pink | 1 | 360 |
Flow Cell Priming Kit contents (EXP-FLP002)

| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (μl) |
|---|---|---|---|---|
| Flush Buffer | FB | Blue | 6 | 1,170 |
| Flush Tether | FLT | Purple | 1 | 200 |
Native Barcoding Expansion 1-12 (EXP-NBD104) and 13-24 (EXP-NBD114) contents
EXP-NBD104 kit contents
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (μl) |
|---|---|---|---|---|
| Native Barcode 01-12 | NB01-12 | White | 12 | 20 |
| Adapter Mix II | AMII | Green | 1 | 40 |
**EXP-NBD114 kit contents** 
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (μl) |
|---|---|---|---|---|
| Native Barcode 13-24 | NB13-24 | White | 12 | 20 |
| Adapter Mix II | AMII | Green | 1 | 40 |
Native Barcoding Expansion 96 (EXP-NBD196) contents
Kits in batches NBD196.10.0007 onwards have barcodes ordered in columns on the plate:
Kits in batches prior to NBD196.10.0007 have barcodes ordered in rows:
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (μl) |
|---|---|---|---|---|
| Native Barcode 01-96 | NB01-96 | - | 1 plate | 40 μl per well |
| Adapter Mix II | AMII | Green | 1 | 70 |
SFB Expansion contents (EXP-SFB001)
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (μl) |
|---|---|---|---|---|
| Short Fragment Buffer | SFB | Grey | 4 | 1,800 |
Adapter Mix II Expansion contents (EXP-AMII001)
| Name | Acronym | Cap colour | No. of tubes | Fill volume per vial (μl) |
|---|---|---|---|---|
| Adapter Mix II | AMII | Green | 2 | 40 |
Adapter Mix II Expansion use
Protocols that use the Native Barcoding Expansions require 5 μl of AMII per reaction. Native Barcoding Expansions EXP-NBD104/NBD114 and EXP-NBD196 contain sufficient AMII for 6 and 12 reactions, respectively (or 12 and 24 reactions when sequencing on Flongle). This assumes that all barcodes are used in one sequencing run.
The Adapter Mix II expansion provides additional AMII for customers who are running subsets of barcodes, and allows a further 12 reactions (24 on Flongle).
Native barcode sequences
Below is the full list of our native barcode (NB01-96) sequences. The first 24 unique barcodes are available in the Native Barcoding Kit 24 V14 (SQK-NBD114.24). The Native Barcoding Kit 96 V14 (SQK-NBD114.96) include the first 24 native barcodes, with the additional 72 unique barcodes. The native barcodes are shipped at 640 nM.
In addition to the barcodes, there are also flanking sequences which add an extra level of context during analysis.
Barcode flanking sequences:
Forward sequence: 5' - AAGGTTAA - barcode - CAGCACCT - 3' Reverse sequence: 5' - GGTGCTG - barcode - TTAACCTTAGCAAT - 3'
Native barcode sequences
| Component | Forward sequence | Reverse sequence |
|---|---|---|
| NB01 | CACAAAGACACCGACAACTTTCTT | AAGAAAGTTGTCGGTGTCTTTGTG |
| NB02 | ACAGACGACTACAAACGGAATCGA | TCGATTCCGTTTGTAGTCGTCTGT |
| NB03 | CCTGGTAACTGGGACACAAGACTC | GAGTCTTGTGTCCCAGTTACCAGG |
| NB04 | TAGGGAAACACGATAGAATCCGAA | TTCGGATTCTATCGTGTTTCCCTA |
| NB05 | AAGGTTACACAAACCCTGGACAAG | CTTGTCCAGGGTTTGTGTAACCTT |
| NB06 | GACTACTTTCTGCCTTTGCGAGAA | TTCTCGCAAAGGCAGAAAGTAGTC |
| NB07 | AAGGATTCATTCCCACGGTAACAC | GTGTTACCGTGGGAATGAATCCTT |
| NB08 | ACGTAACTTGGTTTGTTCCCTGAA | TTCAGGGAACAAACCAAGTTACGT |
| NB09 | AACCAAGACTCGCTGTGCCTAGTT | AACTAGGCACAGCGAGTCTTGGTT |
| NB10 | GAGAGGACAAAGGTTTCAACGCTT | AAGCGTTGAAACCTTTGTCCTCTC |
| NB11 | TCCATTCCCTCCGATAGATGAAAC | GTTTCATCTATCGGAGGGAATGGA |
| NB12 | TCCGATTCTGCTTCTTTCTACCTG | CAGGTAGAAAGAAGCAGAATCGGA |
| NB13 | AGAACGACTTCCATACTCGTGTGA | TCACACGAGTATGGAAGTCGTTCT |
| NB14 | AACGAGTCTCTTGGGACCCATAGA | TCTATGGGTCCCAAGAGACTCGTT |
| NB15 | AGGTCTACCTCGCTAACACCACTG | CAGTGGTGTTAGCGAGGTAGACCT |
| NB16 | CGTCAACTGACAGTGGTTCGTACT | AGTACGAACCACTGTCAGTTGACG |
| NB17 | ACCCTCCAGGAAAGTACCTCTGAT | ATCAGAGGTACTTTCCTGGAGGGT |
| NB18 | CCAAACCCAACAACCTAGATAGGC | GCCTATCTAGGTTGTTGGGTTTGG |
| NB19 | GTTCCTCGTGCAGTGTCAAGAGAT | ATCTCTTGACACTGCACGAGGAAC |
| NB20 | TTGCGTCCTGTTACGAGAACTCAT | ATGAGTTCTCGTAACAGGACGCAA |
| NB21 | GAGCCTCTCATTGTCCGTTCTCTA | TAGAGAACGGACAATGAGAGGCTC |
| NB22 | ACCACTGCCATGTATCAAAGTACG | CGTACTTTGATACATGGCAGTGGT |
| NB23 | CTTACTACCCAGTGAACCTCCTCG | CGAGGAGGTTCACTGGGTAGTAAG |
| NB24 | GCATAGTTCTGCATGATGGGTTAG | CTAACCCATCATGCAGAACTATGC |
| NB25 | GTAAGTTGGGTATGCAACGCAATG | CATTGCGTTGCATACCCAACTTAC |
| NB26 | CATACAGCGACTACGCATTCTCAT | ATGAGAATGCGTAGTCGCTGTATG |
| NB27 | CGACGGTTAGATTCACCTCTTACA | TGTAAGAGGTGAATCTAACCGTCG |
| NB28 | TGAAACCTAAGAAGGCACCGTATC | GATACGGTGCCTTCTTAGGTTTCA |
| NB29 | CTAGACACCTTGGGTTGACAGACC | GGTCTGTCAACCCAAGGTGTCTAG |
| NB30 | TCAGTGAGGATCTACTTCGACCCA | TGGGTCGAAGTAGATCCTCACTGA |
| NB31 | TGCGTACAGCAATCAGTTACATTG | CAATGTAACTGATTGCTGTACGCA |
| NB32 | CCAGTAGAAGTCCGACAACGTCAT | ATGACGTTGTCGGACTTCTACTGG |
| NB33 | CAGACTTGGTACGGTTGGGTAACT | AGTTACCCAACCGTACCAAGTCTG |
| NB34 | GGACGAAGAACTCAAGTCAAAGGC | GCCTTTGACTTGAGTTCTTCGTCC |
| NB35 | CTACTTACGAAGCTGAGGGACTGC | GCAGTCCCTCAGCTTCGTAAGTAG |
| NB36 | ATGTCCCAGTTAGAGGAGGAAACA | TGTTTCCTCCTCTAACTGGGACAT |
| NB37 | GCTTGCGATTGATGCTTAGTATCA | TGATACTAAGCATCAATCGCAAGC |
| NB38 | ACCACAGGAGGACGATACAGAGAA | TTCTCTGTATCGTCCTCCTGTGGT |
| NB39 | CCACAGTGTCAACTAGAGCCTCTC | GAGAGGCTCTAGTTGACACTGTGG |
| NB40 | TAGTTTGGATGACCAAGGATAGCC | GGCTATCCTTGGTCATCCAAACTA |
| NB41 | GGAGTTCGTCCAGAGAAGTACACG | CGTGTACTTCTCTGGACGAACTCC |
| NB42 | CTACGTGTAAGGCATACCTGCCAG | CTGGCAGGTATGCCTTACACGTAG |
| NB43 | CTTTCGTTGTTGACTCGACGGTAG | CTACCGTCGAGTCAACAACGAAAG |
| NB44 | AGTAGAAAGGGTTCCTTCCCACTC | GAGTGGGAAGGAACCCTTTCTACT |
| NB45 | GATCCAACAGAGATGCCTTCAGTG | CACTGAAGGCATCTCTGTTGGATC |
| NB46 | GCTGTGTTCCACTTCATTCTCCTG | CAGGAGAATGAAGTGGAACACAGC |
| NB47 | GTGCAACTTTCCCACAGGTAGTTC | GAACTACCTGTGGGAAAGTTGCAC |
| NB48 | CATCTGGAACGTGGTACACCTGTA | TACAGGTGTACCACGTTCCAGATG |
| NB49 | ACTGGTGCAGCTTTGAACATCTAG | CTAGATGTTCAAAGCTGCACCAGT |
| NB50 | ATGGACTTTGGTAACTTCCTGCGT | ACGCAGGAAGTTACCAAAGTCCAT |
| NB51 | GTTGAATGAGCCTACTGGGTCCTC | GAGGACCCAGTAGGCTCATTCAAC |
| NB52 | TGAGAGACAAGATTGTTCGTGGAC | GTCCACGAACAATCTTGTCTCTCA |
| NB53 | AGATTCAGACCGTCTCATGCAAAG | CTTTGCATGAGACGGTCTGAATCT |
| NB54 | CAAGAGCTTTGACTAAGGAGCATG | CATGCTCCTTAGTCAAAGCTCTTG |
| NB55 | TGGAAGATGAGACCCTGATCTACG | CGTAGATCAGGGTCTCATCTTCCA |
| NB56 | TCACTACTCAACAGGTGGCATGAA | TTCATGCCACCTGTTGAGTAGTGA |
| NB57 | GCTAGGTCAATCTCCTTCGGAAGT | ACTTCCGAAGGAGATTGACCTAGC |
| NB58 | CAGGTTACTCCTCCGTGAGTCTGA | TCAGACTCACGGAGGAGTAACCTG |
| NB59 | TCAATCAAGAAGGGAAAGCAAGGT | ACCTTGCTTTCCCTTCTTGATTGA |
| NB60 | CATGTTCAACCAAGGCTTCTATGG | CCATAGAAGCCTTGGTTGAACATG |
| NB61 | AGAGGGTACTATGTGCCTCAGCAC | GTGCTGAGGCACATAGTACCCTCT |
| NB62 | CACCCACACTTACTTCAGGACGTA | TACGTCCTGAAGTAAGTGTGGGTG |
| NB63 | TTCTGAAGTTCCTGGGTCTTGAAC | GTTCAAGACCCAGGAACTTCAGAA |
| NB64 | GACAGACACCGTTCATCGACTTTC | GAAAGTCGATGAACGGTGTCTGTC |
| NB65 | TTCTCAGTCTTCCTCCAGACAAGG | CCTTGTCTGGAGGAAGACTGAGAA |
| NB66 | CCGATCCTTGTGGCTTCTAACTTC | GAAGTTAGAAGCCACAAGGATCGG |
| NB67 | GTTTGTCATACTCGTGTGCTCACC | GGTGAGCACACGAGTATGACAAAC |
| NB68 | GAATCTAAGCAAACACGAAGGTGG | CCACCTTCGTGTTTGCTTAGATTC |
| NB69 | TACAGTCCGAGCCTCATGTGATCT | AGATCACATGAGGCTCGGACTGTA |
| NB70 | ACCGAGATCCTACGAATGGAGTGT | ACACTCCATTCGTAGGATCTCGGT |
| NB71 | CCTGGGAGCATCAGGTAGTAACAG | CTGTTACTACCTGATGCTCCCAGG |
| NB72 | TAGCTGACTGTCTTCCATACCGAC | GTCGGTATGGAAGACAGTCAGCTA |
| NB73 | AAGAAACAGGATGACAGAACCCTC | GAGGGTTCTGTCATCCTGTTTCTT |
| NB74 | TACAAGCATCCCAACACTTCCACT | AGTGGAAGTGTTGGGATGCTTGTA |
| NB75 | GACCATTGTGATGAACCCTGTTGT | ACAACAGGGTTCATCACAATGGTC |
| NB76 | ATGCTTGTTACATCAACCCTGGAC | GTCCAGGGTTGATGTAACAAGCAT |
| NB77 | CGACCTGTTTCTCAGGGATACAAC | GTTGTATCCCTGAGAAACAGGTCG |
| NB78 | AACAACCGAACCTTTGAATCAGAA | TTCTGATTCAAAGGTTCGGTTGTT |
| NB79 | TCTCGGAGATAGTTCTCACTGCTG | CAGCAGTGAGAACTATCTCCGAGA |
| NB80 | CGGATGAACATAGGATAGCGATTC | GAATCGCTATCCTATGTTCATCCG |
| NB81 | CCTCATCTTGTGAAGTTGTTTCGG | CCGAAACAACTTCACAAGATGAGG |
| NB82 | ACGGTATGTCGAGTTCCAGGACTA | TAGTCCTGGAACTCGACATACCGT |
| NB83 | TGGCTTGATCTAGGTAAGGTCGAA | TTCGACCTTACCTAGATCAAGCCA |
| NB84 | GTAGTGGACCTAGAACCTGTGCCA | TGGCACAGGTTCTAGGTCCACTAC |
| NB85 | AACGGAGGAGTTAGTTGGATGATC | GATCATCCAACTAACTCCTCCGTT |
| NB86 | AGGTGATCCCAACAAGCGTAAGTA | TACTTACGCTTGTTGGGATCACCT |
| NB87 | TACATGCTCCTGTTGTTAGGGAGG | CCTCCCTAACAACAGGAGCATGTA |
| NB88 | TCTTCTACTACCGATCCGAAGCAG | CTGCTTCGGATCGGTAGTAGAAGA |
| NB89 | ACAGCATCAATGTTTGGCTAGTTG | CAACTAGCCAAACATTGATGCTGT |
| NB90 | GATGTAGAGGGTACGGTTTGAGGC | GCCTCAAACCGTACCCTCTACATC |
| NB91 | GGCTCCATAGGAACTCACGCTACT | AGTAGCGTGAGTTCCTATGGAGCC |
| NB92 | TTGTGAGTGGAAAGATACAGGACC | GGTCCTGTATCTTTCCACTCACAA |
| NB93 | AGTTTCCATCACTTCAGACTTGGG | CCCAAGTCTGAAGTGATGGAAACT |
| NB94 | GATTGTCCTCAAACTGCCACCTAC | GTAGGTGGCAGTTTGAGGACAATC |
| NB95 | CCTGTCTGGAAGAAGAATGGACTT | AAGTCCATTCTTCTTCCAGACAGG |
| NB96 | CTGAACGGTCATAGAGTCCACCAT | ATGGTGGACTCTATGACCGTTCAG |
3. Computer requirements and software
MinION Mk1B IT requirements
Sequencing on a MinION Mk1B requires a high-spec computer or laptop to keep up with the rate of data acquisition. For more information, refer to the MinION Mk1B IT requirements document.
MinION Mk1C IT requirements
The MinION Mk1C contains fully-integrated compute and screen, removing the need for any accessories to generate and analyse nanopore data. For more information refer to the MinION Mk1C IT requirements document.
MinION Mk1D IT requirements
Sequencing on a MinION Mk1D requires a high-spec computer or laptop to keep up with the rate of data acquisition. For more information, refer to the MinION Mk1D IT requirements document.
Software for nanopore sequencing
MinKNOW
The MinKNOW software controls the nanopore sequencing device, collects sequencing data and basecalls in real time. You will be using MinKNOW for every sequencing experiment to sequence, basecall and demultiplex if your samples were barcoded.
For instructions on how to run the MinKNOW software, please refer to the MinKNOW protocol.
EPI2ME (optional)
The EPI2ME cloud-based platform performs further analysis of basecalled data, for example alignment to the Lambda genome, barcoding, or taxonomic classification. You will use the EPI2ME platform only if you would like further analysis of your data post-basecalling.
For instructions on how to create an EPI2ME account and install the EPI2ME Desktop Agent, please refer to this link.
Check your flow cell
We highly recommend that you check the number of pores in your flow cell prior to starting a sequencing experiment. This should be done within 12 weeks of purchasing for MinION/GridION/PromethION or within four weeks of purchasing Flongle Flow Cells. Oxford Nanopore Technologies will replace any flow cell with fewer than the number of pores in the table below, when the result is reported within two days of performing the flow cell check, and when the storage recommendations have been followed. To do the flow cell check, please follow the instructions in the Flow Cell Check document.
| Flow cell | Minimum number of active pores covered by warranty |
|---|---|
| Flongle Flow Cell | 50 |
| MinION/GridION Flow Cell | 800 |
| PromethION Flow Cell | 5000 |
4. Reverse transcription
Materials
- Input RNA in 10 mM Tris-HCl, pH 8.0
Consumables
- LunaScript™ RT SuperMix Kit (NEB, cat # E3010)
- 1.5 ml Eppendorf DNA LoBind tubes
- Eppendorf twin.tec® PCR plate 96 LoBind, semi-skirted (Merck, EP0030129504) with heat seals
- Nuclease-free water (e.g. ThermoFisher, AM9937)
Equipment
- P200 pipette and tips
- P2 pipette and tips
- Thermal cycler
- Microfuge
- Ice bucket with ice
Optional equipment
- PCR-Cooler (Eppendorf)
- PCR hood with UV steriliser (optional but recommended to reduce cross-contamination)
Keep the RNA sample on ice as much as possible to prevent nucleolytic degradation, which may affect sensitivity.
In a clean pre-PCR hood, using a stepper pipette, or a multichannel pipette, add 4 µl of LunaScript™ RT SuperMix to a fresh 96-well plate (RT Plate).
Depending on the number of samples, fill each well per column as follows:
| Plate location | X24 samples | X48 samples | X96 samples |
|---|---|---|---|
| Columns | 1-3 | 1-6 | 1-12 |
To each well containing LunaScript reagent of the RT plate, add 16 µl of sample and gently mix by pipetting. If adding less than 16 µl, make up the rest of the volume with nuclease-free water.
Example for X48 samples:
Seal the RT plate and spin down. Return the plate to ice.
Preheat the thermal cycler to 25°C.
Incubate the samples in the thermal cycler using the following program:
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Primer annealing | 25°C | 2 min | 1 |
| cDNA synthesis | 55°C | 10 min | 1 |
| Heat inactivation | 95°C | 1 min | 1 |
| Hold | 4°C | ∞ |
While the reverse transcription reaction is running, prepare the primer pools as described in the next section.
5. PCR and clean-up
Consumables
- SARS-CoV-2 primers (lab-ready at 100 µM, IDT)
- Q5® Hot Start High-Fidelity 2X Master Mix (NEB, cat # M0494)
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Agencourt AMPure XP beads (Beckman Coulter™, A63881)
- Freshly prepared 80% ethanol in nuclease-free water
- 1.5 ml Eppendorf DNA LoBind tubes
- Eppendorf twin.tec® PCR plate 96 LoBind, semi-skirted (Merck, EP0030129504) with heat seals
Equipment
- Microfuge
- Thermal cycler
- Hula mixer (gentle rotator mixer)
- Magnetic rack suitable for 96-well plates
- Multichannel pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
Optional equipment
- Qubit™ fluorometer (or equivalent for QC check)
- Agilent Bioanalyzer (or equivalent)
- PCR hood with UV steriliser (optional but recommended to reduce cross-contamination)
Primer design
To generate tiled PCR amplicons from the SARS-CoV-2 viral cDNA, primers were designed by Josh Quick using Primal Scheme. These primers are designed to generate 400 bp amplicons that overlap by approximately 20 bp. These primer sequences can be found here. Where we show example data outputs in this protocol, the same parameters were used to design primers to the human coronavirus 229E to provide guideline statistics.
We recommend ordering the required primers from IDT in a lab-ready format at 100 µM. However, if primers have been ordered lyophilised, they should be resuspended in water or low-EDTA TE buffer to a final concentration of 100 µM.
We recommend handling the primer stocks and derivatives in a clean template-free PCR hood.
Dilute each 100 µM stock 1 in 10 with nuclease-free water to form a working stock of each pool at 10 µM.
Note: To achieve the desired final concentration of each primer in the pool at 0.015 µM in the PCR reaction, 3.7 µl of the 10 µM working stock is needed for each PCR reaction. Two separate PCR reactions will be performed per sample, one for pool A primers and one for pool B. This results in tiled amplicons that have approximately 20 bp overlap.
In the pre-PCR hood, prepare the following master mixes in Eppendorf DNA LoBind tubes and mix thoroughly as follows:
Per sample:
| Reagent | Pool A | Pool B |
|---|---|---|
| RNase-free water | 3.8 µl | 3.8 µl |
| Primer pool A (10 µM) | 3.7 µl | - |
| Primer pool B (10 µM) | - | 3.7 µl |
| Q5® Hot Start HF 2x Master Mix | 12.5 µl | 12.5 µl |
| Total | 20 µl | 20 µl |
For x24 samples:
| Reagent | Pool A | Pool B |
|---|---|---|
| RNase-free water | 95 µl | 95 µl |
| Primer pool A (10 µM) | 92.5 µl | - |
| Primer pool B (10 µM) | - | 92.5 µl |
| Q5® Hot Start HF 2x Master Mix | 312.5 µl | 312.5 µl |
| Total | 500 µl | 500 µl |
For x48 samples:
| Reagent | Pool A | Pool B |
|---|---|---|
| RNase-free water | 190 µl | 190 µl |
| Primer pool A (10 µM) | 185 µl | - |
| Primer pool B (10 µM) | - | 185 µl |
| Q5® Hot Start HF 2x Master Mix | 625 µl | 625 µl |
| Total | 1000 µl | 1000 µl |
For x96 samples:
| Reagent | Pool A | Pool B |
|---|---|---|
| RNase-free water | 380 µl | 380 µl |
| Primer pool A (10 µM) | 370 µl | - |
| Primer pool B (10 µM) | - | 370 µl |
| Q5® Hot Start HF 2x Master Mix | 1250 µl | 1250 µl |
| Total | 2000 µl | 2000 µl |
Using a stepper pipette or a multichannel pipette, aliquot 20 µl of Pool A and Pool B into a clean 96-well plate(s) as follows:
| Plate location | X24 samples | X48 samples | X96 samples |
|---|---|---|---|
| Columns | Pool A: 1-3 Pool B: 4-6 | Pool A: 1-6 Pool B: 7-12 | Pool A: 1-12 Pool B: 1-12 |
Note: For x96 samples, Pool A is a separate plate to Pool B.
Using a multichannel pipette, transfer 5 µl of each RT reaction from the RT plate to the corresponding well for both Pool A and Pool B of the PCR plate(s). Mix by pipetting the contents of each well up and down.
There should be two PCR reactions per sample.
Example for X48 samples:
Carry forward the negative control from the reverse transcription reaction to monitor cross-contamination events.
We recommend having a single negative for every plate of samples and a standard curve of positive controls.
Seal the plate(s) and spin down briefly.
Incubate using the following program, with the heated lid set to 105°C:
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Initial denaturation | 98°C | 30 sec | 1 |
| Denaturation Annealing and extension | 98°C 65°C | 15 sec 5 min | 25–35 |
| Hold | 4°C | ∞ |
Note: Cycle number should be varied for low or high viral load samples. Guidelines provided by Josh Quick suggest that 25 cycles should be used for Ct 18–21 up to a maximum of 35 cycles for Ct 35, however this has not been tested here.
If available, a clean post-PCR hood should be used for all steps that involve handling amplified material. Decontamination with UV and or DNAzap between sample batches is recommended.
Once PCR is complete, using a multichannel pipette, transfer 25 µl of each well of PCR Pool B to the corresponding well of PCR Pool A and mix by pipetting.
Depending on the number of samples, Pool B columns will correspond to different Pool A columns.
| No. of samples | Pool B column | Corresponding Pool A column |
|---|---|---|
| x24 | 4 5 6 | 1 2 3 |
| x48 | 7 8 9 10 11 12 | 1 2 3 4 5 6 |
| x96 | 1 2 3 4 5 6 7 8 9 10 11 12 | 1 2 3 4 5 6 7 8 9 10 11 12 |
Example for X48 samples:
Resuspend the AMPure XP beads by vortexing.
Add 50 µl of resuspended AMPure XP beads to each well and mix by gently pipetting.
Incubate on a Hula mixer (rotator mixer) for 10 minutes at room temperature.
Prepare 50 ml of fresh 80% ethanol in nuclease-free water.
Spin down the 96-well plate and pellet the beads on a magnet for 5 minutes. Keep the plate on the magnet until the eluate is clear and colourless, and pipette off the supernatant.
Keep the plate on the magnet and wash the beads in each well with 200 µl of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the plate back on the magnet. Pipette off any residual ethanol. Allow to dry for ~ 30 seconds, but do not dry the pellet to the point of cracking.
Remove the plate from the magnetic rack and resuspend each pellet in 15 µl nuclease-free water. Incubate for 2 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless.
Remove and retain 15 µl of eluate containing the DNA library per well, into a clean 96-well plate.
Dispose of the pelleted beads.
Quantify 1 µl of each eluted sample using a Qubit fluorometer.
Store any unused amplified material at -20°C for use in later experiments.
Expected results
During initial method development, it is useful to analyse 1 µl on an Agilent Bioanalyzer chip or an appropriate amount on an agarose gel. The traces below show expected results where a dilution series of coronavirus 229E was spiked into 100 ng of human RNA extracted from GM12878 (primers were designed against the human coronavirus 229E reference genome using Primal Scheme). Here, Qubit hsDNA results and Agilent Bioanalyser (DNA 12000 assay) traces are shown for 30 and 35 cycles of PCR with input concentrations ranging from 10 pg to 0.001 pg in 100 ng human RNA. While not directly comparable to Ct values of a real biological sample, these give a rough approximation of high to low viral titres. A human-only and reverse transcription negative control were also included.
Note: The viral RNA that was used for this spike-in experiment was obtained from ATCC and is total RNA extracted from human cell lines infected with coronavirus 229E. So, 10 pg of spike-in represents a mix of human and viral RNA, spiked into 100 ng of human RNA extracted from GM12878 cells.
Figure 1. DNA yield after PCR and AMPure XP clean-up for decreasing viral input and different PCR cycle numbers in a background of 100 ng human RNA.
For a 400 bp amplicon, approximately 50 ng (~0.2 pmol) is required for the end-prep step. PCR cycles can be adjusted based on initial results to minimise the number of cycles. For samples that provide less than 50 ng total yield, further PCRs may be carried out on the remaining reverse transcription reaction.
Figure 2. Bioanalyser traces of 1 µl of post-PCR cleaned up samples with decreasing input quantities, spiked into 100 ng human RNA amplified with 30 and 35 cycles. RT negative controls and human-only negative controls show no product in the 300–400 bp range.
6. End-prep
Consumables
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- NEBNext® Ultra™ II End Repair/dA-Tailing Module (NEB, E7546)
- 1.5 ml Eppendorf DNA LoBind tubes
- Eppendorf twin.tec® PCR plate 96 LoBind, semi-skirted (Merck, EP0030129504) with heat seals
Equipment
- Multichannel pipette and tips
- P1000 pipette and tips
- P100 pipette and tips
- P10 pipette and tips
- Thermal cycler
- Microfuge
- Ice bucket with ice
For optimal efficiency of the end-prep reaction, use ~200 fmol (50 ng for 400 bp amplicons) of cDNA from the previous step.
We recommended carrying the RT negative control through this step until sequencing.
Determine the volume of the cleaned-up PCR reaction that yields 200 fmol (50 ng) of DNA per sample and aliquot in a clean 96-well plate.
Prepare the NEBNext Ultra II End Repair/dA-Tailing Module reagents according to the manufacturer’s instructions, and place on ice.
For optimal performance, NEB recommend the following:
- Thaw all reagents on ice.
- Flick and/or invert reagent tube to ensure they are well mixed.
- Always spin down tubes before opening for the first time each day.
- The Ultra II End prep buffer and FFPE DNA Repair buffer may have a little precipitate. Allow the mixture to come to room temperature and pipette the buffer up and down several times to break up the precipitate, followed by vortexing the tube for several seconds to ensure the reagent is thoroughly mixed.
- The FFPE DNA repair buffer may have a yellow tinge and is fine to use if yellow.
Make up each sample per well to 12.5 µl using nuclease-free water.
Prepare the following end-prep master mix in a 1.5 ml Eppendorf DNA LoBind tube and mix thoroughly.
| Reagent | Volume per sample | Volume x24 samples | Volume x48 samples | Volume x96 samples |
|---|---|---|---|---|
| NEBNext Ultra II End Prep Reaction Buffer | 1.75 µl | 52.5 µl | 105 µl | 210 µl |
| NEBNext Ultra II End Prep Enzyme Mix | 0.75 µl | 22.5 µl | 45 µl | 90 µl |
| Total | 2.5 µl | 75 µl | 150 µl | 300 µl |
Using a stepper pipette, or a multichannel pipette, aliquot 2.5 µl of the end-prep master mix per sample (End-prep Plate) and keep on ice.
Depending on the number of samples, aliquot into each well of the columns as follows:
| Plate location | X24 samples | X48 samples | X96 samples |
|---|---|---|---|
| Columns | 1-3 | 1-6 | 1-12 |
Seal the plate(s) and spin down briefly.
Using a thermal cycler, incubate at 20°C for 5 minutes and 65°C for 5 minutes.
Take forward the end-prepped DNA into the native barcode ligation step. However, at this point it is also possible to store the sample at 4°C overnight.
7. Native barcode ligation
Materials
- Native Barcodes (NB01-24)
- Native Barcodes (NB01-NB96)
- S Fragment Buffer (SFB)
Consumables
- Freshly prepared 80% ethanol in nuclease-free water
- 1.5 ml Eppendorf DNA LoBind tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- NEB Blunt/TA Ligase Master Mix (NEB, M0367)
Equipment
- Magnetic rack suitable for 96-well plates
- Thermal cycler
- Hula mixer (gentle rotator mixer)
- Vortex mixer
- Ice bucket with ice
- Microfuge
- P1000 pipette and tips
- P100 pipette and tips
- P10 pipette and tips
Optional equipment
- Qubit™ fluorometer (or equivalent for QC check)
To monitor cross-contamination events, we recommend that the RT negative control is carried through this process and a barcode is used to sequence this control.
Thaw the native barcodes at room temperature. Use one barcode per sample. Individually mix the barcodes by pipetting, spin down, and place them on ice.
Thaw the tube of Short Fragment Buffer (SFB) at room temperature, mix by vortexing, spin down and place on ice.
Select a unique barcode for every sample to be run.
Using a stepper pipette, or a multichannel pipette, make up the Native Barcode Ligation Plate in a clean 96-well plate. Add the reagents in the following order per well:
| Reagent | Volume per well for up to x24 samples | Volume per well for x25—x96 samples |
|---|---|---|
| Nuclease-free water | 6 µl | 3 µl |
| End-prepped DNA Note: Transfer to the corresponding well | 1.5 µl | 0.75 µl |
| Native barcode Note: Transfer to the corresponding well | 2.5 µl | 1.25 µl |
| Blunt/TA Ligation Master Mix | 10 µl | 5 µl |
| Total | 20 µl | 10 µl |
Depending on the number of samples, aliquot to each well of the columns:
| Plate location | x24 samples | x48 samples | x96 samples |
|---|---|---|---|
| Columns | 1-3 | 1-6 | 1-12 |
Mix the contents thoroughly by pipetting.
Seal the plate(s) and spin down briefly.
Using a thermal cycler, incubate at 20°C for 20 mins and at 65°C for 10 mins.
Pool the barcoded samples.
- For up to x24 samples, we expect ~20 µl per sample.
- For x25—x96 samples, we expect ~10 µl per sample.
| x24 samples | x48 samples | x96 samples | |
|---|---|---|---|
| Total volume | ~480 µl | ~480 µl | ~960 µl |
Take forward 480 µl of the pooled barcoded samples.
For x96 samples, there will be enough pooled reaction remaining for a second library to be prepared. This can be stored at –20°C for later use.
Resuspend the AMPure XP beads by vortexing.
Add 192 µl of resuspended AMPure XP beads to the 480 µl of pooled barcoded reaction and mix by pipetting.
Incubate on a Hula mixer (rotator mixer) for 10 minutes at room temperature.
Prepare 500 µl of fresh 80% ethanol in nuclease-free water.
Spin down the sample and pellet the beads on a magnet for 5 minutes. Keep the tube on the magnet until the eluate is clear and colourless, and pipette off the supernatant.
Wash the beads by adding 700 μl Short Fragment Buffer (SFB). Flick the beads to resuspend, then return the tube to the magnetic rack and allow the beads to pellet. Remove the supernatant using a pipette and discard.
Repeat the previous step.
Keep the tube on the magnet and wash the beads with 100 µl of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 35 µl nuclease-free water. Incubate for 2 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless.
Remove and retain 35 µl of eluate into a clean 1.5 ml Eppendorf DNA LoBind tube.
Quantify 1 µl of eluted sample using a Qubit fluorometer - recovery aim 2 ng/μl.
Take forward 30-50 ng of pooled barcoded samples in 30 µl into the adapter ligation and clean-up step.
8. Adapter ligation and clean-up
Materials
- Elution Buffer (EB)
- S Fragment Buffer (SFB)
- Adapter Mix II (AMII)
Consumables
- NEBNext Quick Ligation Module (NEB, E6056)
- Agencourt AMPure XP beads (Beckman Coulter™, A63881)
- 1.5 ml Eppendorf DNA LoBind tubes
Equipment
- Microfuge
- Magnetic separation rack
- Vortex mixer
- Hula mixer (gentle rotator mixer)
Optional equipment
- Qubit™ fluorometer (or equivalent for QC check)
Adapter Mix II Expansion use
Protocols that use the Native Barcoding Expansions require 5 μl of AMII per reaction. Native Barcoding Expansions EXP-NBD104/NBD114 and EXP-NBD196 contain sufficient AMII for 6 and 12 reactions, respectively (or 12 and 24 reactions when sequencing on Flongle). This assumes that all barcodes are used in one sequencing run.
The Adapter Mix II expansion provides additional AMII for customers who are running subsets of barcodes, and allows a further 12 reactions (24 on Flongle).
Thaw the Elution Buffer (EB), Short Fragment Buffer (SFB), and NEBNext Quick Ligation Reaction Buffer (5x) at room temperature and mix by vortexing. Then spin down and place on ice. Check the contents of each tube are clear of any precipitate.
Spin down the T4 Ligase and the Adapter Mix II (AMII), and place on ice.
Taking the pooled and barcoded DNA, perform adapter ligation as follows, mixing by flicking the tube between each sequential addition.
| Reagent | Volume |
|---|---|
| Pooled barcoded sample (30-50 ng) | 30 µl |
| Adapter Mix II (AMII) | 5 µl |
| NEBNext Quick Ligation Reaction Buffer (5X) | 10 µl |
| Quick T4 DNA Ligase | 5 µl |
| Total | 50 µl |
Ensure the components are thoroughly mixed by pipetting, and spin down.
Incubate the reaction for 10 minutes at room temperature.
The next clean-up step uses Short Fragment Buffer (SFB) and not 80% ethanol to wash the beads. The use of ethanol will be detrimental to the sequencing reaction.
Resuspend the AMPure XP beads by vortexing.
Add 20 µl of resuspended AMPure XP beads to the reaction and mix by pipetting.
Incubate on a Hula mixer (rotator mixer) for 10 minutes at room temperature.
Spin down the sample and pellet the beads on a magnet for 5 minutes. Keep the tube on the magnet until the eluate is clear and colourless, and pipette off the supernatant.
Wash the beads by adding 125 μl Short Fragment Buffer (SFB). Flick the beads to resuspend, then return the tube to the magnetic rack and allow the beads to pellet. Keep the tube on the magnet until the eluate is clear and colourless. Remove the supernatant using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual supernatant. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet by pipetting in 15 µl Elution Buffer (EB). Spin down and incubate for 5 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Remove and retain 15 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads
Quantify 1 µl of eluted sample using a Qubit fluorometer.
We recommend loading ~15 ng of final prepared library onto a flow cell.
Loading more than the recommend loading input can have a detrimental effect on output. Dilute the library in Elution Buffer (EB) if required.
The prepared library is used for loading onto the flow cell. Store the library on ice or at 4°C until ready to load.
Library storage recommendations
We recommend storing libraries in Eppendorf DNA LoBind tubes at 4°C for short-term storage or repeated use, for example, re-loading flow cells between washes. For single use and long-term storage of more than 3 months, we recommend storing libraries at -80°C in Eppendorf DNA LoBind tubes.
If quantities allow, the library may be diluted in Elution Buffer (EB) for splitting across multiple flow cells.
Additional buffer for doing this can be found in the Sequencing Auxiliary Vials expansion (EXP-AUX001), available to purchase separately. This expansion also contains additional vials of Sequencing Buffer (SQB) and Loading Beads (LB), required for loading the libraries onto flow cells.
9. Priming and loading the SpotON flow cell
Materials
- Flow Cell Priming Kit (EXP-FLP002)
- Loading Beads (LB)
- Sequencing Buffer (SQB)
Consumables
- 1.5 ml Eppendorf DNA LoBind tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
Equipment
- MinION Mk1B or Mk1C
- SpotON Flow Cell
- MinION/GridION Flow Cell Light Shield
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
Priming and loading a flow cell
We recommend all new users watch the 'Priming and loading your flow cell' video before your first run.
Thaw the Sequencing Buffer (SQB), Loading Beads (LB), Flush Tether (FLT) and one tube of Flush Buffer (FB) at room temperature before mixing the reagents by vortexing, and spin down at room temperature.
To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Open the MinION device lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check instructions in the MinKNOW protocol for more information.
Slide the flow cell priming port cover clockwise to open the priming port.
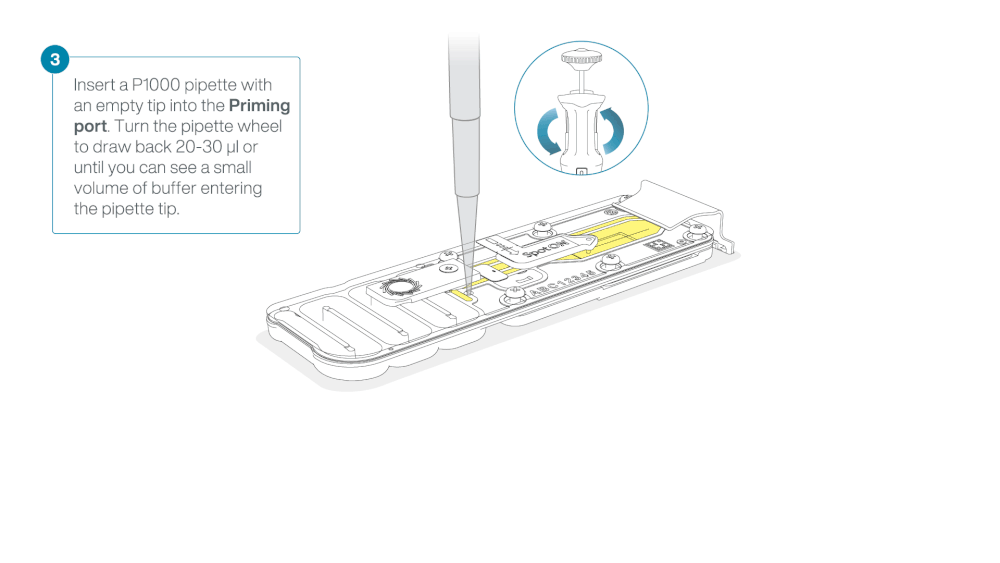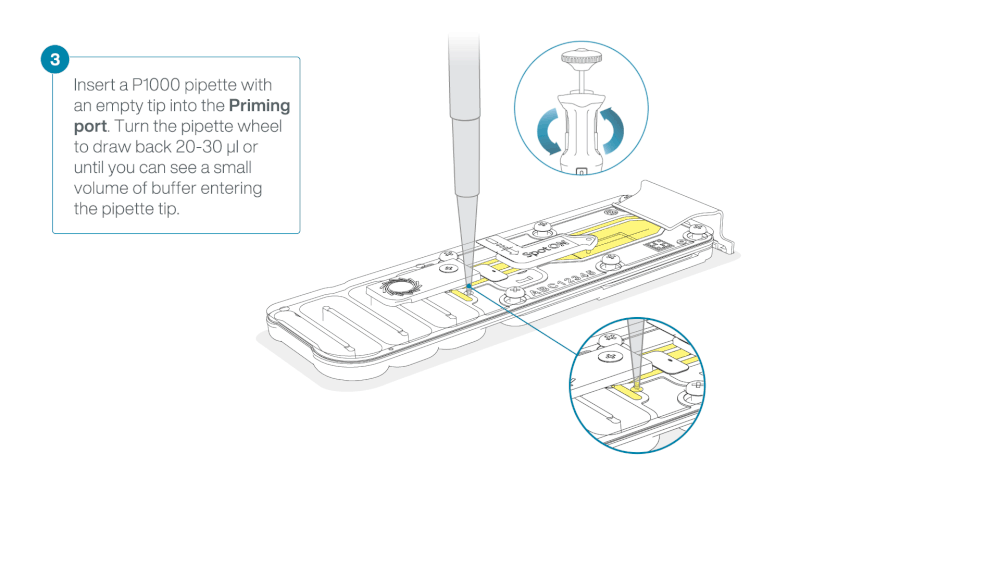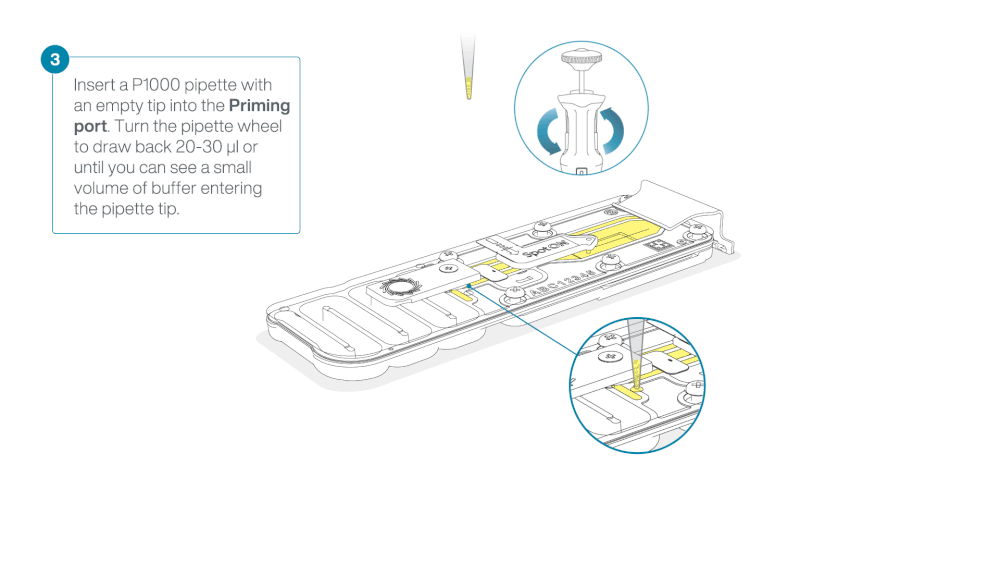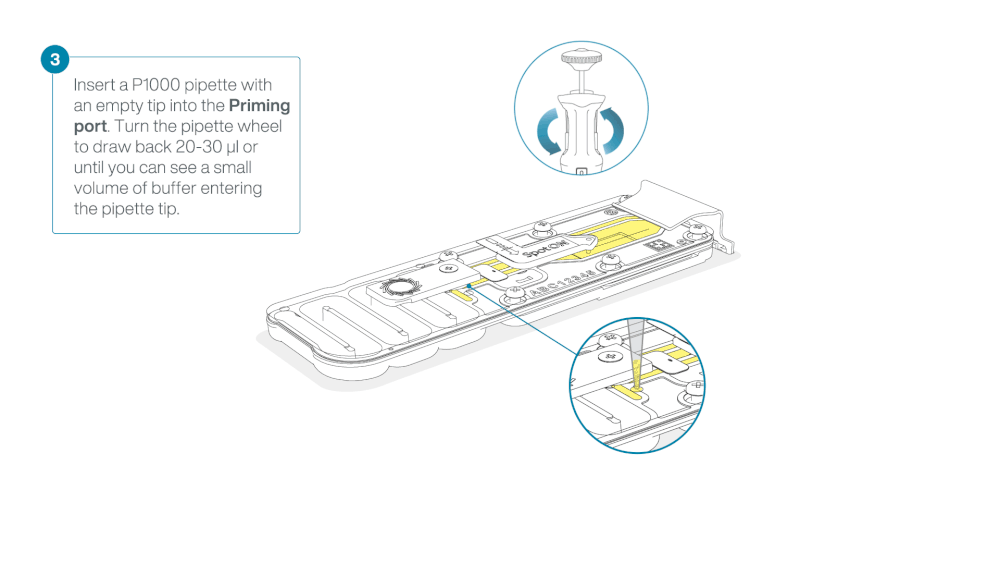
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 µl, to draw back 20-30 µl, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the Loading Beads (LB) by pipetting.
The Loading Beads (LB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SQB) | 37.5 µl |
| Loading Beads (LB), mixed immediately before use | 25.5 µl |
| DNA library | 12 µl |
| Total | 75 µl |
Note: Load the library onto the flow cell immediately after adding the Sequencing Buffer (SQB) and Loading Beads (LB) because the fuel in the buffer will start to be consumed by the adapter.
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port and close the priming port.

Install the light shield on your flow cell as soon as library has been loaded for optimal sequencing output.
We recommend leaving the light shield on the flow cell when library is loaded, including during any washing and reloading steps. The shield can be removed when the library has been removed from the flow cell.
Place the light shield onto the flow cell, as follows:
Carefully place the leading edge of the light shield against the clip. Note: Do not force the light shield underneath the clip.
Gently lower the light shield onto the flow cell. The light shield should sit around the SpotON cover, covering the entire top section of the flow cell.

The MinION Flow Cell Light Shield is not secured to the flow cell and careful handling is required after installation.
Close the device lid and set up a sequencing run on MinKNOW.
10. Data acquisition and basecalling
Overview of nanopore data analysis
For a full overview of nanopore data analysis, which includes options for basecalling and post-basecalling analysis, please refer to the Data Analysis document.
How to start sequencing
The sequencing device control, data acquisition and real-time basecalling are carried out by the MinKNOW software. Please ensure MinKNOW is installed on your computer or device. There are multiple options for how to carry out sequencing:
1. Data acquisition and basecalling in real-time using MinKNOW on a computer
Follow the instructions in the MinKNOW protocol beginning from the "Starting a sequencing run" section until the end of the "Completing a MinKNOW run" section.
2. Data acquisition and basecalling in real-time using the MinION Mk1B/Mk1D device
Follow the instructions in the MinION Mk1B user manual or the MinION Mk1D user manual.
3. Data acquisition and basecalling in real-time using the MinION Mk1C device
Follow the instructions in the MinION Mk1C user manual.
4. Data acquisition and basecalling in real-time using the GridION device
Follow the instructions in the GridION user manual.
5. Data acquisition and basecalling in real-time using the PromethION device
Follow the instructions in the PromethION user manual or the PromethION 2 Solo user manual.
6. Data acquisition using MinKNOW on a computer and basecalling at a later time using MinKNOW
Follow the instructions in the MinKNOW protocol beginning from the "Starting a sequencing run" section until the end of the "Completing a MinKNOW run" section. When setting your experiment parameters, set the Basecalling tab to OFF. After the sequencing experiment has completed, follow the instructions in the Post-run analysis section of the MinKNOW protocol.
Required settings in MinKNOW
When setting the sequencing parameters in MinKNOW, in the Basecalling set barcoding as Enabled, and in the barcoding options, toggle Barcode both ends, Mid-read barcodes and Override minimum mid barcoding score to ON and set Minimum mid barcoding score to 50. Optional: basecalling and/or demultiplexing of sequences can be performed using the stand-alone Guppy software.

11. Downstream analysis and expected results
Recommended analysis pipeline
The recommended workflows for the bioinformatics analyses are provided by the ARTIC network and are documented on their web pages at https://artic.network/ncov-2019/ncov2019-bioinformatics-sop.html.
The reference guided genome assembly and variant calling are also performed according to the bioinformatics protocol provided by the ARTIC network. Their best practices guide uses the software contained within the FieldBioinformatics project on GitHub.
This workflow uses only the basecalled FASTQ files to perform a high-quality reference-guided assembly of the SARS-CoV-2 genome. Sequenced reads are re-demultiplexed with the requirement that reads must contain a barcode at both ends of the sequence (this only applies to the Classic and Eco PCR tiling of SARS-CoV-2 protocols but not the Rapid Barcoding PCR tiling of SARS-CoV-2), and must not contain internal barcodes. The reads are mapped to the reference genome, primer sequences are excluded and the consensus sequence is polished. The Medaka software is used to call single-nucleotide variants while the ARTIC software reports the high-quality consensus sequence from the workflow.
To further simplify the installation of the coronavirus bioinformatics protocols, the workflows have been packaged into two EPI2ME products
The FieldBioinformatics workflow for SARS-CoV-2 sequence analysis is provided as a Jupyter notebook tutorial in the EPI2ME Labs software. The coronavirus workflow has been augmented to include additional steps that help with the quality control of individual libraries, and aid in the presentation of summary statistics and the final sets of called variants.
The FieldBioinformatics workflow for SARS-CoV-2 sequence analysis is also provided as an EPI2ME workflow – this provides a more accessible interface to a bioinformatics workflow and the provided cloud-based analysis also performs some secondary interpretation by preparing an additional report using the Nextclade software.
Expected results
Here, results are shown based on human coronavirus 229E spiked into 100 ng of human RNA derived from GM12878 cell line. 10 pg–0.001 pg of viral RNA obtained from ATCC was spiked into the human RNA and human-only and reverse transcription negative controls were carried through the prep to sequencing. Every sample underwent 30 and 35 cycles of PCR to determine sensitivity and specificity guidelines, as well as the expected amplicon drop-out rate for each sample.
Note: The viral RNA from ATCC is generated from cell lines infected with human coronavirus 229E. The RNA supplied is total RNA extracted from the cell lines and includes both human and viral RNA. Therefore, the levels of sensitivity are likely to be higher than those reported here.
Sample balancing
The graph below shows the expected sequence balancing if the protocol is followed. Here, equal masses went into the end-prep and native barcode ligation prior to pooling by equal mass for adapter ligation.
Figure 3. Number of reads per sample after native barcode demultiplexing in MinKNOW. All 14 samples were run on a single flow cell.
On-target rate
Sequences from each demultiplexed sample were aligned to the human coronavirus 229E genome using minimap2. The proportion of primary alignments per sample are reported below.
Figure 4. Proportion of reads for each sample aligning to the human coronavirus 229E reference genome.
Assessment of negative controls
After 12 hours of sequencing, the number of reads from the negative control samples aligning to the viral reference genome is shown in the graph below and is compared with the absolute number of sequences aligning to the lowest input (0.001 pg).
Figure 5. Absolute number of reads aligning to the human coronavirus 229E reference genome in the negative controls compared with the lowest input of viral RNA. Sequencing was carried out for 12 hours to pick up low levels of sequences assigned to barcodes representing these samples.
Target coverage for different PCR cycles and viral load
To assess the impact of PCR dropout with lowering input viral load and increasing PCR cycles, Mosdepth was used to calculate the proportion of the viral genome covered to different depth levels. These numbers were calculated after 12 hours of sequencing with 14 samples multiplexed.
Figure 6. Coverage and depth of the human coronavirus 229E genome for different input quantities of viral RNA and different cycle numbers after 12 hours of sequencing on a single flow cell.
How much sequencing is required?
This is unknown in real clinical samples. The graph below can be used to determine the proportion of the genome that could be covered to a given depth with different numbers of reads (30 cycles) at different input amounts in a background of 100 ng human RNA. Note: this is absolute depth.
Figure 7. Subsampled sequences to give an indication of the depth of sequencing achievable covering different amounts of the human coronavirus 229E genome. Input quantities and cycle number titrations show that high cycle numbers should be avoided where possible to minimise amplicon drop out.
This protocol provides amplification of low copy number viral genomes in a tiled method with low off-target amplification and minimal cross-contamination between samples. With <60 copies per reaction (0.001 pg viral input) in 100 ng background human RNA, under ideal circumstances, one should expect to cover >75% of the targeted genome at a depth of 200X within under 50,000 reads in the samples with the lowest viral titre and <20,000 reads in those with a higher viral titre.
12. Flow cell reuse and returns
Materials
- Flow Cell Wash Kit (EXP-WSH004)
After your sequencing experiment is complete, if you would like to reuse the flow cell, please follow the Flow Cell Wash Kit protocol and store the washed flow cell at +2°C to +8°C.
The Flow Cell Wash Kit protocol is available on the Nanopore Community.
We recommend you to wash the flow cell as soon as possible after you stop the run. However, if this is not possible, leave the flow cell on the device and wash it the next day.
Alternatively, follow the returns procedure to send the flow cell back to Oxford Nanopore.
Instructions for returning flow cells can be found here.
If you encounter issues or have questions about your sequencing experiment, please refer to the Troubleshooting Guide that can be found in the online version of this protocol.
13. Issues during DNA/RNA extraction and library preparation
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Low sample quality
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low DNA purity (Nanodrop reading for DNA OD 260/280 is <1.8 and OD 260/230 is <2.0–2.2) | The DNA extraction method does not provide the required purity | The effects of contaminants are shown in the Contaminants document. Please try an alternative extraction method that does not result in contaminant carryover. Consider performing an additional SPRI clean-up step. |
| Low RNA integrity (RNA integrity number <9.5 RIN, or the rRNA band is shown as a smear on the gel) | The RNA degraded during extraction | Try a different RNA extraction method. For more info on RIN, please see the RNA Integrity Number document. Further information can be found in the DNA/RNA Handling page. |
| RNA has a shorter than expected fragment length | The RNA degraded during extraction | Try a different RNA extraction method. For more info on RIN, please see the RNA Integrity Number document. Further information can be found in the DNA/RNA Handling page. We recommend working in an RNase-free environment, and to keep your lab equipment RNase-free when working with RNA. |
Low DNA recovery after AMPure bead clean-up
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low recovery | DNA loss due to a lower than intended AMPure beads-to-sample ratio | 1. AMPure beads settle quickly, so ensure they are well resuspended before adding them to the sample. 2. When the AMPure beads-to-sample ratio is lower than 0.4:1, DNA fragments of any size will be lost during the clean-up. |
| Low recovery | DNA fragments are shorter than expected | The lower the AMPure beads-to-sample ratio, the more stringent the selection against short fragments. Please always determine the input DNA length on an agarose gel (or other gel electrophoresis methods) and then calculate the appropriate amount of AMPure beads to use. 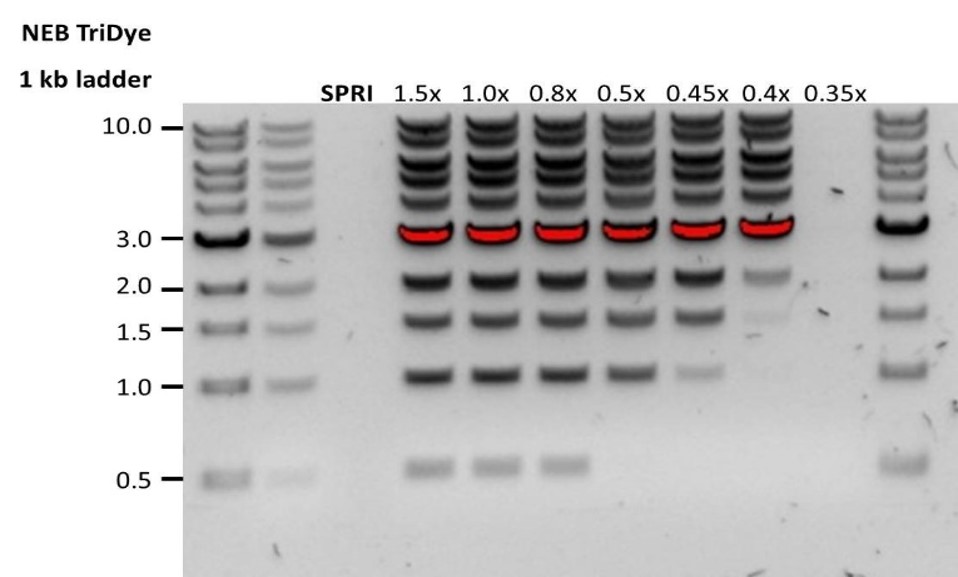 |
| Low recovery after end-prep | The wash step used ethanol <70% | DNA will be eluted from the beads when using ethanol <70%. Make sure to use the correct percentage. |
14. Issues during the sequencing run
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Fewer pores at the start of sequencing than after Flow Cell Check
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | An air bubble was introduced into the nanopore array | After the Flow Cell Check it is essential to remove any air bubbles near the priming port before priming the flow cell. If not removed, the air bubble can travel to the nanopore array and irreversibly damage the nanopores that have been exposed to air. The best practice to prevent this from happening is demonstrated in this video. |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | The flow cell is not correctly inserted into the device | Stop the sequencing run, remove the flow cell from the sequencing device and insert it again, checking that the flow cell is firmly seated in the device and that it has reached the target temperature. If applicable, try a different position on the device (GridION/PromethION). |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | Contaminations in the library damaged or blocked the pores | The pore count during the Flow Cell Check is performed using the QC DNA molecules present in the flow cell storage buffer. At the start of sequencing, the library itself is used to estimate the number of active pores. Because of this, variability of about 10% in the number of pores is expected. A significantly lower pore count reported at the start of sequencing can be due to contaminants in the library that have damaged the membranes or blocked the pores. Alternative DNA/RNA extraction or purification methods may be needed to improve the purity of the input material. The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
MinKNOW script failed
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Script failed" | Restart the computer and then restart MinKNOW. If the issue persists, please collect the MinKNOW log files and contact Technical Support. If you do not have another sequencing device available, we recommend storing the flow cell and the loaded library at 4°C and contact Technical Support for further storage guidance. |
Pore occupancy below 40%
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Pore occupancy <40% | Not enough library was loaded on the flow cell | Ensure you load the recommended amount of good quality library in the relevant library prep protocol onto your flow cell. Please quantify the library before loading and calculate mols using tools like the Promega Biomath Calculator, choosing "dsDNA: µg to pmol" |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and sequencing adapters did not ligate to the DNA | Make sure to use the NEBNext Quick Ligation Module (E6056) and Oxford Nanopore Technologies Ligation Buffer (LNB, provided in the sequencing kit) at the sequencing adapter ligation step, and use the correct amount of each reagent. A Lambda control library can be prepared to test the integrity of the third-party reagents. |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and ethanol was used instead of LFB or SFB at the wash step after sequencing adapter ligation | Ethanol can denature the motor protein on the sequencing adapters. Make sure the LFB or SFB buffer was used after ligation of sequencing adapters. |
| Pore occupancy close to 0 | No tether on the flow cell | Tethers are adding during flow cell priming (FLT/FCT tube). Make sure FLT/FCT was added to FB/FCF before priming. |
Shorter than expected read length
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Shorter than expected read length | Unwanted fragmentation of DNA sample | Read length reflects input DNA fragment length. Input DNA can be fragmented during extraction and library prep. 1. Please review the Extraction Methods in the Nanopore Community for best practice for extraction. 2. Visualise the input DNA fragment length distribution on an agarose gel before proceeding to the library prep. 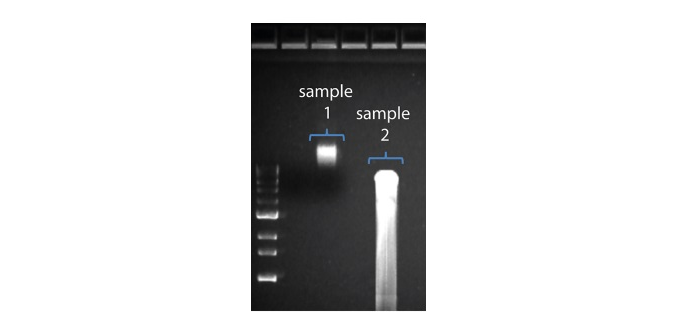 In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented. In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented.3. During library prep, avoid pipetting and vortexing when mixing reagents. Flicking or inverting the tube is sufficient. |
Large proportion of unavailable pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
Large proportion of unavailable pores (shown as blue in the channels panel and pore activity plot) 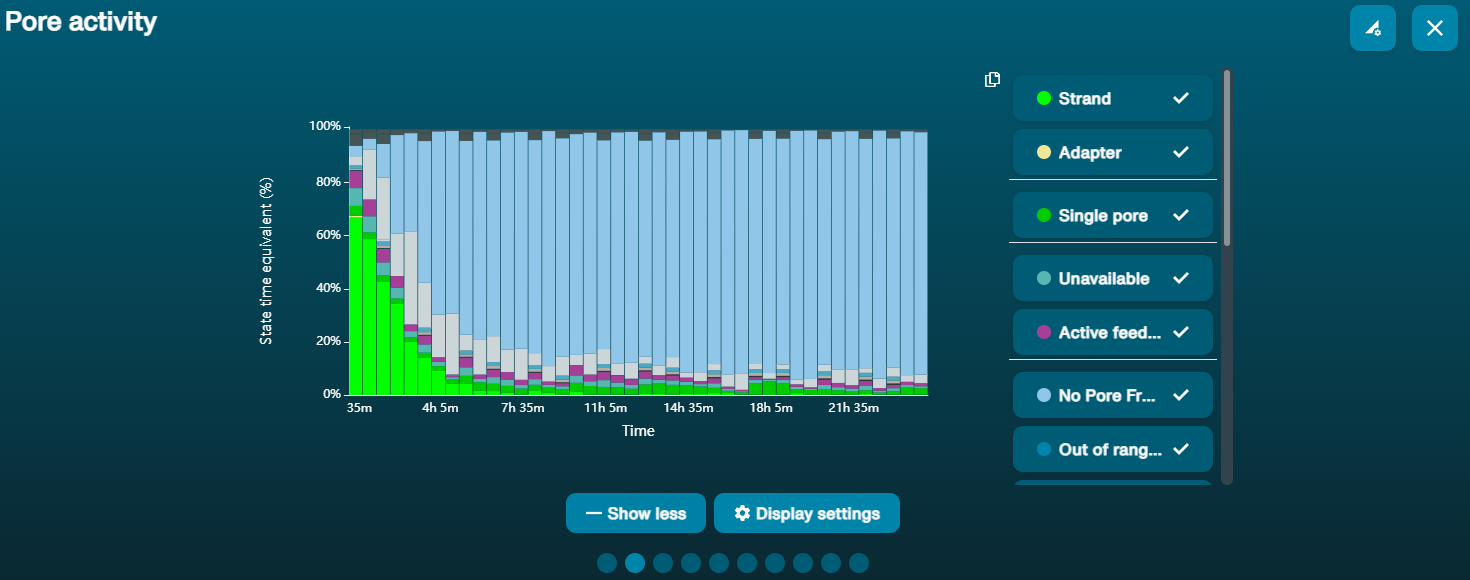 The pore activity plot above shows an increasing proportion of "unavailable" pores over time. The pore activity plot above shows an increasing proportion of "unavailable" pores over time. | Contaminants are present in the sample | Some contaminants can be cleared from the pores by the unblocking function built into MinKNOW. If this is successful, the pore status will change to "sequencing pore". If the portion of unavailable pores stays large or increases: 1. A nuclease flush using the Flow Cell Wash Kit (EXP-WSH004) can be performed, or 2. Run several cycles of PCR to try and dilute any contaminants that may be causing problems. |
Large proportion of inactive pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Large proportion of inactive/unavailable pores (shown as light blue in the channels panel and pore activity plot. Pores or membranes are irreversibly damaged) | Air bubbles have been introduced into the flow cell | Air bubbles introduced through flow cell priming and library loading can irreversibly damage the pores. Watch the Priming and loading your flow cell video for best practice |
| Large proportion of inactive/unavailable pores | Certain compounds co-purified with DNA | Known compounds, include polysaccharides, typically associate with plant genomic DNA. 1. Please refer to the Plant leaf DNA extraction method. 2. Clean-up using the QIAGEN PowerClean Pro kit. 3. Perform a whole genome amplification with the original gDNA sample using the QIAGEN REPLI-g kit. |
| Large proportion of inactive/unavailable pores | Contaminants are present in the sample | The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
Reduction in sequencing speed and q-score later into the run
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Reduction in sequencing speed and q-score later into the run | For Kit 9 chemistry (e.g. SQK-LSK109), fast fuel consumption is typically seen when the flow cell is overloaded with library (please see the appropriate protocol for your DNA library to see the recommendation). | Add more fuel to the flow cell by following the instructions in the MinKNOW protocol. In future experiments, load lower amounts of library to the flow cell. |
Temperature fluctuation
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Temperature fluctuation | The flow cell has lost contact with the device | Check that there is a heat pad covering the metal plate on the back of the flow cell. Re-insert the flow cell and press it down to make sure the connector pins are firmly in contact with the device. If the problem persists, please contact Technical Services. |
Failed to reach target temperature
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Failed to reach target temperature" | The instrument was placed in a location that is colder than normal room temperature, or a location with poor ventilation (which leads to the flow cells overheating) | MinKNOW has a default timeframe for the flow cell to reach the target temperature. Once the timeframe is exceeded, an error message will appear and the sequencing experiment will continue. However, sequencing at an incorrect temperature may lead to a decrease in throughput and lower q-scores. Please adjust the location of the sequencing device to ensure that it is placed at room temperature with good ventilation, then re-start the process in MinKNOW. Please refer to this link for more information on MinION temperature control. |
Guppy – no input .fast5 was found or basecalled
| Observation | Possible cause | Comments and actions |
|---|---|---|
| No input .fast5 was found or basecalled | input_path did not point to the .fast5 file location | The --input_path has to be followed by the full file path to the .fast5 files to be basecalled, and the location has to be accessible either locally or remotely through SSH. |
| No input .fast5 was found or basecalled | The .fast5 files were in a subfolder at the input_path location | To allow Guppy to look into subfolders, add the --recursive flag to the command |
Guppy – no Pass or Fail folders were generated after basecalling
| Observation | Possible cause | Comments and actions |
|---|---|---|
| No Pass or Fail folders were generated after basecalling | The --qscore_filtering flag was not included in the command | The --qscore_filtering flag enables filtering of reads into Pass and Fail folders inside the output folder, based on their strand q-score. When performing live basecalling in MinKNOW, a q-score of 7 (corresponding to a basecall accuracy of ~80%) is used to separate reads into Pass and Fail folders. |
Guppy – unusually slow processing on a GPU computer
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Unusually slow processing on a GPU computer | The --device flag wasn't included in the command | The --device flag specifies a GPU device to use for accelerate basecalling. If not included in the command, GPU will not be used. GPUs are counted from zero. An example is --device cuda:0 cuda:1, when 2 GPUs are specified to use by the Guppy command. |







