VolTRAX V2b (VTX_2004_v1_revW_13May2025)
Protocol
VolTRAX V2b V VTX_2004_v1_revW_13May2025
For Research Use Only
FOR RESEARCH USE ONLY
Contents
VolTRAX V2b overview
- 1. Introduction to VolTRAX V2b
- 2. Laboratory equipment and consumables
- 3. Computer requirements for using VolTRAX V2b
Installing the VolTRAX V2b software
Running a VolTRAX Configuration Kit (VCK-V2003b)
Running a VolTRAX Sequencing Kit (VSK-VSK004)
- 7. Overview of the VolTRAX Sequencing Kit
- 8. Run a VolTRAX Sequencing Kit
- 9. Priming and loading the SpotON flow cell
Running a VolTRAX Multiplex Kit (VSK-VMK004)
- 10. An overview of the VolTRAX Multiplex Kit
- 11. Run a VolTRAX Multiplex Kit
- 12. Priming and loading the SpotON flow cell
Running a VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001)
- 13. Overview of the VolTRAX PCR Tiling 1-12 COVID-19 Kit
- 14. Run a VolTRAX PCR Tiling 1-12 COVID-19 Kit
- 15. Priming and loading the SpotON flow cell
Running a VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001) with customisable PCR parameters
Overview
For Research Use Only
1. Introduction to VolTRAX V2b
VolTRAX upgrade path
In early Q2 2022, the VolTRAX V2 devices in field began to be phased out and replaced by VolTRAX V2b. The VolTRAX V2b Starter Packs contain:
- 1 VolTRAX V2b device
- 1 VolTRAX Configuration Kit (VCK-V2003b), including two blue cartridges
- 4 VolTRAX Cartridge Packs (VCT-V2002), each pack contains three blue cartridges
- 4 VolTRAX Sequencing Kits (VSK-VSK004), each kit is sufficient for three reactions
- 6 MinION Flow Cells
- 1 USB3.1 / USBC cable
Also in 2022, five new products were introduced that can be purchased individually:
- VolTRAX Sequencing Kit (VSK-VSK004)
- VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001)
- VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001)
- VolTRAX Multiplex Kit (VSK-VMK004)
- VolTRAX Cartridge Packs (VCT-V2002D), containing blue cartridges
Introduction to VolTRAX V2b
The VolTRAX V2b is a portable, multi-purpose device designed to automatically prepare DNA or RNA libraries ready for analysis on a nanopore sequencing platform. The device is compact and USB-powered, to allow portability of library preparation and sequencing in a laboratory or non-laboratory environment.

The VolTRAX V2b with a disposable cartridge inserted.
Simple, automated, and portable library preparation of VolTRAX V2b
The VolTRAX V2b device along with the cartridge allows a user to choose a library preparation protocol, load their sample with supplied reagents and press go. The device prepares the library following a series of steps programmed by the software.
The device includes heating elements for incubations and PCR, and magnetic elements for bead-based separations. These features combine with the VolTRAX capabilities to precisely move, mix and incubate droplets to enable both basic and complex sample preparation methods to be carried out with a click of a button.
VolTRAX V2b is simple to use and needs only minutes of hands-on time to prepare a library.
2. Laboratory equipment and consumables
Materials
- VolTRAX Configuration Kit (VCK-V2003b)
- VolTRAX Sequencing Kit (VSK-VSK004)
- VolTRAX Multiplex Kit (VSK-VMK004)
- VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001)
- VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001)
- VolTRAX Cartridge Pack (VCT-V2002B)
Consumables
- VolTRAX cartridge
- MinION Flow Cell
- 1.5 ml Eppendorf DNA LoBind tubes
- 0.2 ml thin-walled PCR tubes
Equipment
- VolTRAX V2b
- P20 pipette and tips
- P10 pipette and tips
- MinION
VolTRAX V2b pipette tip compatibility
We have found that certain pipette tips give better results during the reagent loading and library extraction. Using the wrong tip type can lead to sample loss. The Mettler Toledo (Rainin™) UNV 10 µl tips have been verified as having the correct geometry for loading reagents on the VolTRAX cartridge. We recommend the Mettler Toledo (Rainin™) UNV 20 µl tips for extracting the prepared library at the end of the run.
No third-party reagents are required for running a VolTRAX Sequencing Kit.
Sample requirements to prepare high-quality libraries (see individual kit sections for details)
The VolTRAX Sequencing Kit (VSK-VSK004) and VolTRAX Multiplex Kit (VSK-VMK004) protocols for VolTRAX V2b require clean, extracted DNA that is of high molecular weight, accurately-quantified and free from contaminants and detergents. The DNA should be prepared in 10 mM Tris-HCl, pH 8.0.
The VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001) and VolTRAX RT-PCR Sequencing Kit 1-12 protocol requires total RNA in 10 mM Tris-HCl, pH 8.0, extracted from samples that have been screened by a suitable qPCR assay. Samples must have a Ct value of 30 or less, and be free from contaminants and detergents.
Contaminants
Contaminants can impede the function of the library preparation chemistry for VolTRAX V2b. For information about the impact of contaminants on reaction efficiency, see the Contaminants Know-How piece.
3. Computer requirements for using VolTRAX V2b
Computer requirements for VolTRAX V2b
VolTRAX V2b is driven by the VolTRAX software, which is responsible for the control of the device and consumable cartridge array.
VolTRAX V2b computer requirement is less demanding than a MinION™. If you are currently running a MinION Mk1B on Windows, or you plan to use a MinION Mk1B with VolTRAX, the computer requirements for your MinION Mk1B should be sufficient to operate the VolTRAX.
If you plan to purchase a system dedicated to VolTRAX V2b, the minimum requirements are:
| Component | Minimum specification |
|---|---|
| Operating system | Windows 10 macOS and Linux are in development. |
| Memory/RAM | 2 GB RAM |
| CPU | 64-bit dual core processor |
| Storage/hard drive | > 128 GB SSD |
| User account privilege level | Local administrator |
| Ports | USB TYPE C, 5 V, 3 A † |
| Antivirus software | Installation highly recommended |
| Internet connection | Appropriate firewall settings are required for downloading the VolTRAX software. |
† Only the USB TYPE C – Gen 3.1 (I or II) or higher – 5 V, 3 A can provide the full range of functionalities on VolTRAX V2b.
The VolTRAX status is displayed by LEDs
To ensure users can easily determine their VolTRAX status; a series of LEDs are included and described below.
Under-side LEDs:
The LEDs shown in the figure are indicators of the power supplied to the device.

- Red - This indicates that the USB Type-C cable is correctly inserted into the port on the device. This is for Development use only.
- Red - This LED signals that 900 mA are being supplied to the device; this is the least powerful type of USB-Type C or 3.1 Type I/II port. The device will operate basic protocols, but will not be able to perform thermal cycling required for PCR-based library preparations.
- Blue - This LED signals that 1.5 A are being supplied to the device; this is a commonly occurring power supply of USB-Type C ports. The device will operate basic protocols, but will not be able to perform thermal cycling required for PCR-based library preparations.
- Green - This LED signals 3 A are being supplied to the device; this is the requirement for the full capabilities of the VolTRAX V2B device.
- Blue - This LED signals power to the device.
- Blue - This LED signals power to the device.
- These are for use by Oxford Nanopore Technologies.
It is particularly important that LED 4 (green) is illuminated, if the user requires the thermal cycler to prepare their library. If the LED is not on, 3 A are not being supplied to the device.
To check that the laptop to be used with the VolTRAX has a port that supplies 3 A and that the power rating is set to 3 A, contact your IT Department.
Internally at Oxford Nanopore Technologies, we have used USB-power meters as a way of measuring the current provided by a USB port when a laptop is running off charge or plugged in. Users may wish to look into these in advance of their VolTRAX devices arriving.
Firewall settings for Oxford Nanopore Technologies' devices
The Oxford Nanopore Technologies software will require access to the AWS IP ranges currently listed here:
http://docs.aws.amazon.com/general/latest/gr/aws-ip-ranges.html
Access to the following IP addresses is also needed:
178.79.175.200
96.126.99.215
Updates
VolTRAX software is under rapid development and we ask users to keep up-to-date with the latest software revisions.
Latest software versions will be available on our software downloads page.
Microsoft Windows updates
It is essential to check the settings for Windows updates. Windows Updates Automatic restarts need to be disabled and it is recommended that Windows Update installs are set to occur on shutdown only.
4. Installing the VolTRAX V2b software on Windows
You must not plug the VolTRAX V2b into the designated laptop before the VolTRAX Client is installed, operational, and instructs you to.
To download the VolTRAX Client to run a VolTRAX V2b, follow the instructions below:
The most current version of VolTRAX Client is available from the Software Downloads page.
Note: You will need Administrative rights to install the VolTRAX Client and VolTRAX firmware. The VolTRAX Client is currently only supported on Windows 10 operating systems.
The executable (.exe) will be downloaded to your default downloads folder. Unless otherwise specified, this is the 'Downloads' folder on the C:\ drive.
Double-click on the VolTRAX installer executable to begin installation, and follow the prompts.
Once installation is complete, double-click the VolTRAX Client shortcut on the desktop.
The VolTRAX Client will load a window. Click "Start VolTRAX".

The Windows Firewall may interrupt installation.
The Windows Defender Firewall, or similar, may block the download of some of the features of the application.
If this is the case, contact your IT department to enable the full download of the VolTRAX Client.

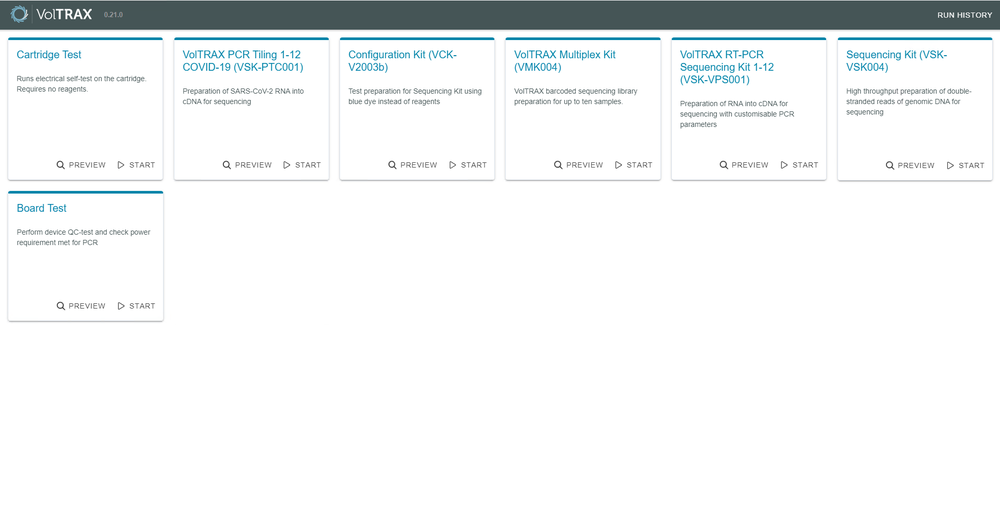
The VolTRAX Client will load up the protocol selector screen, where the VolTRAX Client will lead you through the requirements to execute the selected method.
The Protocol Selector screen provides the supported protocol options that can be run with VolTRAX V2b.
Default output folder
For both types of installation (whether the software is installed for "Anyone who uses this computer" or "Only for me"), by default the output data is logged to:
C:\Users\[username]\AppData\Local\VolTRAX\run\
5. Running a Cartridge Test for VolTRAX V2b
Materials
- VolTRAX Configuration Kit (VCK-V2003b)
Equipment
- VolTRAX V2b
The Cartridge Test may be used to pre-screen cartridges prior to use. The Cartridge Test is also performed as part of each kit workflow.
Once the software is running, plug in your VolTRAX device. Click the "Start" button.
You will be presented with the following options for protocols to run. Select "Cartridge Test" to start the protocol and follow the instructions in the software.

Please refer to the videos displayed in the VolTRAX UI, which demonstrate how to perform each step in the protocol.
If the Cartridge Test fails:
- Removing the cartridge as shown in the animation in the GUI, re-insert the cartridge and repeat the Cartridge Test.
- If the Cartridge Test fails a second time, contact Customer Support at support@nanoporetech.com

Cancelling a run
To cancel a run, select Cancel in the top right corner of the GUI.
The user is able to look through all the steps of the selected protocol before confirming cancellation.
The run will stop immediately and return the user to the "Start VolTRAX" page. Another run can be started immediately.
Note: The cartridge must be replaced if reagents have been input during a cancelled run. If no reagent has been inserted, the cartridge can be used again.
Run history
The UI provides a run history option to view previously-run protocols, which can be accessed from the link in the top right corner.

Clicking Open on any of the protocols will navigate the user to the relevant data folder in Windows File Explorer:

6. Running a VolTRAX Configuration Kit
Materials
- VolTRAX Configuration Kit (VCK-V2003b)
Equipment
- VolTRAX V2b
- P20 pipette and tips
- P10 pipette and tips
The VolTRAX Configuration Kit (VCK-V2003b) enables the practice of loading and extracting material from the VolTRAX V2b cartridge.
Below is an overview of the configuration experiment using a VolTRAX V2b with the Configuration Kit (VCK-V2003b).
An overview of VolTRAX V2b configuration
1. Loading a protocol
- Use the protocol selector in the UI to load the VCK-V2003b protocol.
- When prompted, connect your VolTRAX V2b device, press Continue and await connection.
2. System QC - Cartridge Test
- When prompted, connect a cartridge to the VolTRAX device and press Continue to proceed to the Cartridge Test.
3. Running and loading VCS
- Follow instructions and advice on the equipment required and pipetting technique
- Prime the cartridge as instructed by the UI
- Load the VolTRAX Configuration Solution (VCS) and the VX AMPure XP Config (VXC) into the ports as instructed by the UI
- The solutions will be manipulated to indicate what the user should expect during a run
4. Extracting the VolTRAX Configuration Solution from the cartridge
- Follow the extraction instructions, using UI as a visual guide, to slowly extract the VCS
- Discard the extracted VCS
Start the VolTRAX software. You will be presented with the following options for protocols to run. Select "Configuration Kit" to start the protocol and follow the instructions in the software.

Protocol preview
Once an option is selected from the menu, the UI will show a preview of the steps involved in library preparation. The example below is for the Configuration Kit.

When prompted by the UI, connect your VolTRAX device to the computer. When it is connected, you will then be prompted to insert a cartridge into the device.
Prime the cartridge as instructed in the UI by twisting the Priming Fluid container anti-clockwise and removing the foil tab to allow the fluid to enter the cartridge.
Select Continue when each step is completed.
Follow the steps as described in the UI, loading VolTRAX Configuration Solution and VX AMPure XP Config into the wells instead of the reagents stated.
Select Continue after each step. After all the steps have been completed, the library prep process will start automatically.
Please refer to the videos displayed in the VolTRAX UI, which demonstrate how to perform each step in the protocol.
Loading solutions into the VolTRAX V2b cartridge
The VolTRAX V2b device can measure solution volumes loaded into the cartridge. The GUI displays a coloured-pixel grid to show:
- Blue: Volume of solution too low. You will need to load more solution to proceed with the protocol.
- Green: Correct volume of solution.
- Orange: slightly overfilled. You will still be able to proceed with the protocol.
- Red: solution overfilled. You will need to cancel the experiment and start again.

At the end of the experiment, the VolTRAX Configuration Solution can be extracted from the E port of the VolTRAX V2b cartridge. This can be discarded.
Cancelling a run
To cancel a run, select Cancel in the top right corner of the GUI.
The user is able to look through all the steps of the selected protocol before confirming cancellation.
The run will stop immediately and return the user to the "Start VolTRAX" page. Another run can be started immediately.
Note: The cartridge must be replaced if reagents have been input during a cancelled run. If no reagent has been inserted, the cartridge can be used again.
Run history
The UI provides a run history option to view previously-run protocols, which can be accessed from the link in the top right corner.

Clicking Open on any of the protocols will navigate the user to the relevant data folder in Windows File Explorer:

7. Overview of the VolTRAX Sequencing Kit
The VSK-VSK004 DNA library preparation kit
The VolTRAX V2b provides an automated process for preparing a DNA library. The chemistry of the library preparation is the same as for manual library preparation for a MinION or GridION sequencing experiment.
Below is an overview of the library preparation stages using a VolTRAX V2b, blue cartridge and the VolTRAX Sequencing Kit (VSK-VSK004).
Overview of the VolTRAX Sequencing Kit protocol
1. Loading a protocol
- Use the protocol selector in the UI to load the VSK-VSK004 protocol.
- When prompted, connect your VolTRAX V2b device, press Continue and await connection.
2. System QC - Cartridge Test
- When prompted, connect a cartridge to the VolTRAX device and press Continue to proceed to the Cartridge Test.
3. Loading the Priming Fluid and reagents into the cartridge
- Prime the cartridge by twisting the oil container and removing the foil tab.
- The UI will recommend the quality, concentration, buffer and Absorbance (A) readings for your input DNA. These are stated at the stage of 'Reagent Loading' later in this protocol.
- Follow the instructions on the equipment required and pipetting technique.
- Load the appropriate reagents and extracted DNA into the ports as instructed by the UI.
- The reagents will be manipulated by the VolTRAX V2b to prepare the library for nanopore sequencing.
4. Extracting the library from the cartridge
- Follow the extraction instructions, using UI as a visual guide, to slowly extract the library from the cartridge, then transfer it into an Eppendorf tube.
- Prepare the library for loading onto the flow cell.
VolTRAX Sequencing Kit (VSK-VSK004) contents
| Name | Acronym | Cap colour | Number of vials | Fill volume (μl) |
|---|---|---|---|---|
| VX Fragmentation Mix | VFA | Amber | 1 | 25 |
| EDTA | EDTA | Clear | 1 | 20 |
| VX AMPure XP Beads | VXP | Pink | 1 | 45 |
| VX Long Fragment Buffer | VLF | Orange | 1 | 40 |
| VX Rapid Adapter T | VRA T | Green | 1 | 25 |
| VX Elution Buffer | VEB | Black | 1 | 65 |
| VX Running Buffer | VRB | Red | 1 | 250 |
| Flush Buffer | FB | Blue | 3 | 1,170 |
| Flush Tether | FLT | White cap, purple label | 1 | 200 |
VSK-VSK004 chemistry
The VSK-VSK004 library preparation protocol applies our rapid chemistry, with a bead-based purification step.
8. Run a VolTRAX Sequencing Kit
Materials
- 9.5 μl DNA in 10 mM Tris-HCl, pH 8.0 at a DNA concentration of 50 ng/μl (475 ng total)
- VolTRAX Sequencing Kit (VSK-VSK004)
- VolTRAX Cartridge Pack (VCT-V2002B)
Consumables
- 0.2 ml thin-walled PCR tubes
- Qubit™ dsDNA HS Assay Kit (ThermoFisher, Q32851)
Equipment
- VolTRAX V2b
- P20 pipette and tips
- P10 pipette and tips
Optional equipment
- Qubit™ fluorometer (or equivalent for QC check)
VolTRAX V2b pipette tip compatibility
We have found that certain pipette tips give better results during the reagent loading and library extraction. Using the wrong tip type can lead to sample loss. The Mettler Toledo (Rainin™) UNV 10 µl tips have been verified as having the correct geometry for loading reagents on the VolTRAX cartridge. We recommend the Mettler Toledo (Rainin™) UNV 20 µl tips for extracting the prepared library at the end of the run.
Start the VolTRAX software. You will be presented with the following options for protocols to run. Select "Sequencing Kit" to start the protocol and follow the instructions in the software.

Protocol preview
Once an option is selected from the menu, the UI will show a preview of the steps involved in library preparation. The example below is for the Configuration Kit.

When prompted by the UI, connect your VolTRAX device to the computer. When it is connected, you will then be prompted to insert a cartridge into the device.
Prime the cartridge as instructed in the UI by twisting the Priming Fluid container anti-clockwise and removing the foil tab to allow the fluid to enter the cartridge.
Select Continue when each step is completed.
Please refer to the videos displayed in the VolTRAX UI, which demonstrate how to perform each step in the protocol.
Loading solutions into the VolTRAX V2b cartridge
The VolTRAX V2b device can measure solution volumes loaded into the cartridge. The GUI displays a coloured-pixel grid to show:
- Blue: Volume of solution too low. You will need to load more solution to proceed with the protocol.
- Green: Correct volume of solution.
- Orange: slightly overfilled. You will still be able to proceed with the protocol.
- Red: solution overfilled. You will need to cancel the experiment and start again.

Wait for the library prep to finish.
A timer can be found on the right hand side of the UI, indicating the remaining time.
Cancelling a run
To cancel a run, select Cancel in the top right corner of the GUI.
The user is able to look through all the steps of the selected protocol before confirming cancellation.
The run will stop immediately and return the user to the "Start VolTRAX" page. Another run can be started immediately.
Note: The cartridge must be replaced if reagents have been input during a cancelled run. If no reagent has been inserted, the cartridge can be used again.
At the end of the experiment, extract the prepared library from the E port of the VolTRAX cartridge. Dispense the library into a 0.2 ml PCR tube for preparation of the sequencing mix, as discussed in the following section.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Run history
The UI provides a run history option to view previously-run protocols, which can be accessed from the link in the top right corner.

Clicking Open on any of the protocols will navigate the user to the relevant data folder in Windows File Explorer:

9. Priming and loading the SpotON flow cell
Materials
- Flush Buffer (FB)
- Flush Tether (FLT)
Consumables
- MinION Flow Cell
- 1.5 ml Eppendorf DNA LoBind tubes
Equipment
- P1000 pipette and tips
- P100 pipette and tips
Open the MinION Mk1B lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check instructions in the MinKNOW protocol for more information.
Slide the priming port cover clockwise to open the priming port.
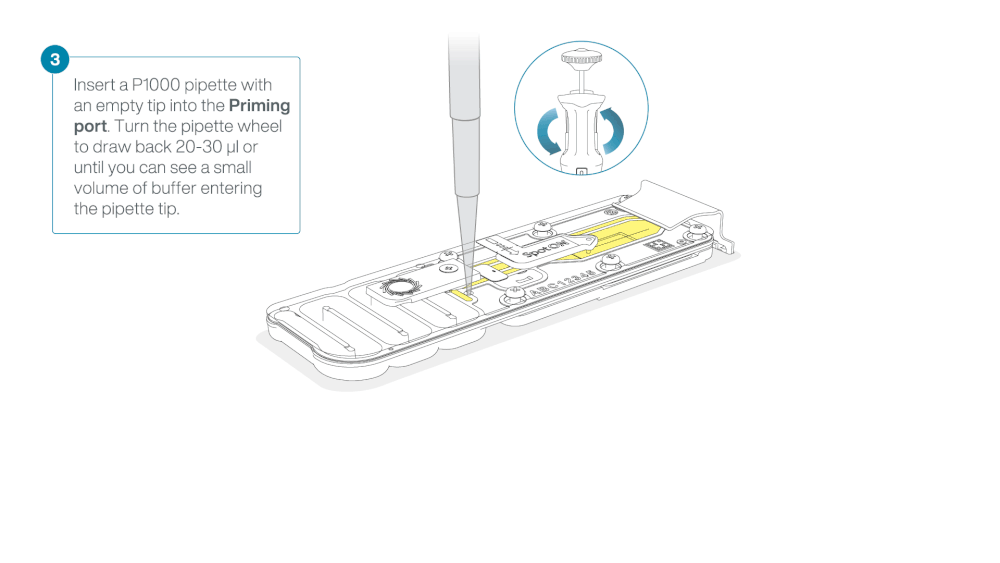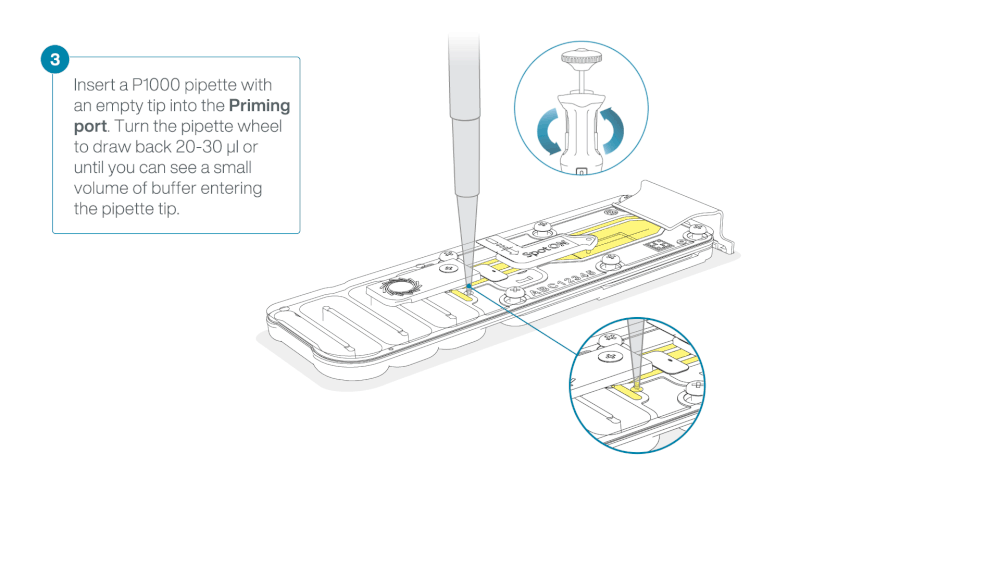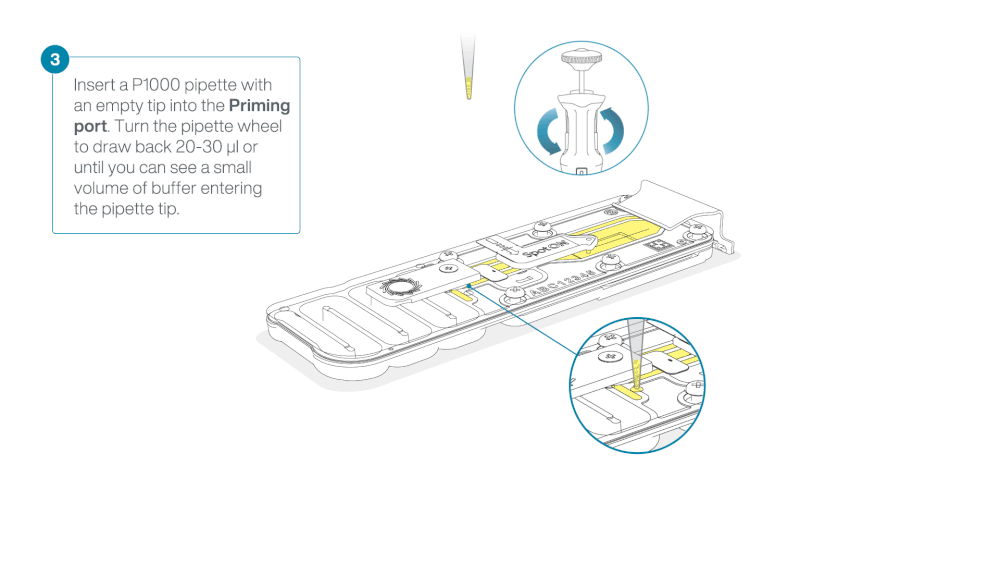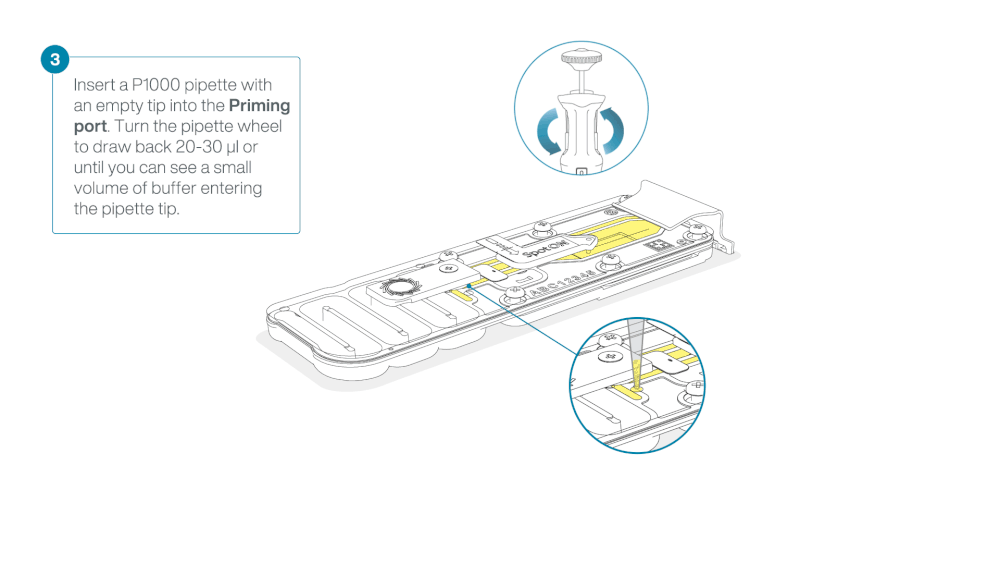
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles (a few µls).
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 ul, to draw back 20-30 ul, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Thoroughly mix the contents of the VX Running Buffer (VRB) tube by pipetting up and down.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| VX Running Buffer (VRB) | 64 µl |
| DNA library | 11 µl |
| Total | 75 µl |
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the priming port and close the lid of the sequencing device.
You are ready to begin your sequencing experiment.
Please refer to the MinKNOW protocol for instructions on setting up your sequencing experiment.
10. An overview of the VolTRAX Multiplex Kit
The VSK-VMK004 DNA library preparation kit
The VolTRAX V2b provides an automated process for preparing a DNA library. The chemistry of the library preparation is the same as for manual library preparation for a MinION or GridION sequencing experiment.
Below is an overview of the library preparation stages using a VolTRAX V2b and the VolTRAX Multiplex Kit (VSK-VMK004).
VolTRAX Multiplex Kit (VSK-VMK004) contents
| Name | Acronym | Number of vials | Cap colour | Fill volume per vial (µl) |
|---|---|---|---|---|
| VolTRAX Buffer Solution | VBS | 1 | Orange | 100 |
| EDTA | EDTA | 1 | Clear | 20 |
| VX AMPure XP Beads | VXP | 1 | Pink | 45 |
| VX S Fragment Buffer | VSF | 1 | White | 30 |
| VX Rapid Adapter T | VRA T | 1 | Green | 25 |
| VX Elution Buffer | VEB | 1 | Black | 65 |
| VX Running Buffer | VRB | 1 | Red | 250 |
| VX BC Frag Mix 01-10 | VB01-10 | 10 | Amber | 20 |
| Flush Buffer | FB | 3 | Blue | 1,170 |
| Flush Tether | FLT | 1 | Purple | 200 |
VolTRAX Multiplex Kit barcode sequences
| Component | Sequence |
|---|---|
| VB01 | AAGAAAGTTGTCGGTGTCTTTGTG |
| VB02 | TCGATTCCGTTTGTAGTCGTCTGT |
| VB03 | GAGTCTTGTGTCCCAGTTACCAGG |
| VB04 | TTCGGATTCTATCGTGTTTCCCTA |
| VB05 | CTTGTCCAGGGTTTGTGTAACCTT |
| VB06 | TTCTCGCAAAGGCAGAAAGTAGTC |
| VB07 | GTGTTACCGTGGGAATGAATCCTT |
| VB08 | TTCAGGGAACAAACCAAGTTACGT |
| VB09 | AACTAGGCACAGCGAGTCTTGGTT |
| VB10 | AAGCGTTGAAACCTTTGTCCTCTC |
VolTRAX Multiplex Kit chemistry
The VSK-VMK004 library preparation protocol uses our rapid chemistry, with a bead-based clean-up step. The kit contains ten barcodes for multiplexing up to ten samples in one library preparation.
An overview of the VolTRAX Multiplex Kit protocol
1. Loading a protocol
- Use the protocol selector in the UI to load the VSK-VMK004 protocol.
- When prompted, connect your VolTRAX V2b device, press Continue and await connection.
2. System QC - Cartridge Test
- When prompted, connect a cartridge to the VolTRAX device and press Continue to proceed to the Cartridge Test.
3. Loading the Priming Fluid and reagents into the cartridge
- Prime the cartridge by twisting the oil container and removing the foil tab.
- The UI will recommend the quality, concentration, buffer and Absorbance (A) readings for your input DNA. These are stated at the stage of 'Reagent Loading' later in this protocol.
- Follow the instructions on the equipment required and pipetting technique.
- Load the appropriate reagents and extracted DNA into the ports as instructed by the UI.
- The reagents will be manipulated by the VolTRAX V2b to prepare the library for nanopore sequencing.
4. Extracting the library from the cartridge
- Follow the extraction instructions, using UI as a visual guide, to slowly extract the library from the cartridge, then transfer it into an Eppendorf tube.
- Prepare the library for loading onto the flow cell.
11. Run a VolTRAX Multiplex Kit
Materials
- VolTRAX Multiplex Kit (VSK-VMK004)
- VolTRAX Cartridge Pack (VCT-V2002B)
- 1.8 μl of extracted gDNA in 10 mM Tris-HCl, pH 8.0 at a DNA concentration of 50 ng/μl (90 ng total) for each sample to be barcoded
Consumables
- 0.2 ml thin-walled PCR tubes
- Qubit™ dsDNA HS Assay Kit (ThermoFisher, Q32851)
Equipment
- VolTRAX V2b
- P20 pipette and tips
- P2 pipette and tips
- Qubit™ fluorometer (or equivalent for QC check)
VolTRAX V2b pipette tip compatibility
We have found that certain pipette tips give better results during the reagent loading and library extraction. Using the wrong tip type can lead to sample loss. The Mettler Toledo (Rainin™) UNV 10 µl tips have been verified as having the correct geometry for loading reagents on the VolTRAX cartridge. We recommend the Mettler Toledo (Rainin™) UNV 20 µl tips for extracting the prepared library at the end of the run.
Start the VolTRAX software. You will be presented with the following options for protocols to run. Select "VolTRAX Multiplex Kit" to start the protocol and follow the instructions in the software.

Protocol preview
Once an option is selected from the menu, the UI will show a preview of the steps involved in library preparation. The example below is for the Configuration Kit.

When prompted by the UI, connect your VolTRAX device to the computer. When it is connected, you will then be prompted to insert a cartridge into the device.
Once the software is running, plug in your VolTRAX device. Click the "Start" button.
You will be presented with five options for test experiments to run. Select "Multiplexing Kit" to start the workflow.

Insert the cartridge firmly into the device until you hear a click. From here on, do not remove the cartridge until the end of the experiment.
Prime the cartridge as instructed in the UI by twisting the Priming Fluid container anti-clockwise and removing the foil tab to allow the fluid to enter the cartridge.
Select Continue when each step is completed.
Please refer to the videos displayed in the VolTRAX UI, which demonstrate how to perform each step in the protocol.
Loading solutions into the VolTRAX V2b cartridge
The VolTRAX V2b device can measure solution volumes loaded into the cartridge. The GUI displays a coloured-pixel grid to show:
- Blue: Volume of solution too low. You will need to load more solution to proceed with the protocol.
- Green: Correct volume of solution.
- Orange: slightly overfilled. You will still be able to proceed with the protocol.
- Red: solution overfilled. You will need to cancel the experiment and start again.

Select the number of samples you are preparing and assign sample aliases to barcodes.

Once you have entered the required details, click Confirm. You will be taken to the Sample Label Check page where you can confirm that the details are correct.

If you are running fewer than 10 samples, you can adjust the "Sample number". Add VBS (VolTRAX Buffer Solution) to wells that will be without a sample.

Wait for the library prep to finish.
A timer can be found on the right hand side of the UI, indicating the remaining time.
Cancelling a run
To cancel a run, select Cancel in the top right corner of the GUI.
The user is able to look through all the steps of the selected protocol before confirming cancellation.
The run will stop immediately and return the user to the "Start VolTRAX" page. Another run can be started immediately.
Note: The cartridge must be replaced if reagents have been input during a cancelled run. If no reagent has been inserted, the cartridge can be used again.
At the end of the experiment, extract the prepared library from the E port of the VolTRAX cartridge. Dispense the library into a 0.2 ml PCR tube for preparation of the sequencing mix, as discussed in the following section.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Run history
The UI provides a run history option to view previously-run protocols, which can be accessed from the link in the top right corner.

Clicking Open on any of the protocols will navigate the user to the relevant data folder in Windows File Explorer:

12. Priming and loading the SpotON flow cell
Materials
- Flush Buffer (FB)
- Flush Tether (FLT)
Consumables
- MinION/GridION Flow Cell
- 1.5 ml Eppendorf DNA LoBind tubes
Equipment
- P1000 pipette and tips
- P100 pipette and tips
Open the MinION Mk1B lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.

Slide the priming port cover clockwise to open the priming port.

How to prime and load the SpotON Flow Cell
Priming and loading: The steps for priming and loading the SpotON Flow Cell. Written instructions are given below. The library is loaded dropwise without putting the pipette tip firmly into the port.
Take care to avoid introducing any air during pipetting.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles (a few µls).
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 ul, to draw back 20-30 ul, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.

To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the VX Running Buffer (VRB) tube by pipetting up and down.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| VX Running Buffer (VRB) | 64 µl |
| DNA library | 11 µl |
| Total | 75 µl |
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the priming port and close the lid of the sequencing device.
You are ready to begin your sequencing experiment.
Please refer to the MinKNOW protocol for instructions on setting up your sequencing experiment.
13. Overview of the VolTRAX PCR Tiling 1-12 COVID-19 Kit
The VSK-PTC001 RNA library preparation kit
The VolTRAX PCR Tiling 1-12 COVID-19 Kit generates tiled PCR amplicons from the SARS-CoV-2 viral RNA for whole genome sequencing using the VolTRAX V2b platform to improve consistency in library preparation due to the precise fluid handling of the device.
This kit provides all the reagents required to generate reverse-transcribed and PCR-amplified material from SARS-CoV-2 RNA samples using VolTRAX automation to prepare the library, without the need to purchase further components. The PCR tiling generated overlapping amplicons across the whole SARS-CoV-2 genome before barcoding and pooling multiple samples together for sequencing.
Below is an overview of the library preparation stages using a VolTRAX V2b, blue cartridges and the VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001).
Overview of the VolTRAX PCR Tiling 1-12 COVID-19 protocol
1. Loading a protocol
- Use the protocol selector in the UI to load the VSK-PTC001 protocol.
- When prompted, connect your VolTRAX V2b device, press Continue and await connection.
2. System QC - Cartridge Test
- When prompted, connect a cartridge to the VolTRAX device and press Continue to proceed to the Cartridge Test.
3. Loading the Priming Fluid and reagents into the cartridge
- Follow the instructions on the equipment required and pipetting technique.
- Prime the cartridge by twisting the oil container and removing the foil tab.
- Load the appropriate reagents and extracted RNA into the ports as instructed by the UI.
- The reagents will be manipulated by the VolTRAX V2b to prepare the library for nanopore sequencing.
4. Extracting the library from the cartridge
- Follow the extraction instructions, using UI as a visual guide, to slowly extract the library from the cartridge, then transfer it into an Eppendorf tube.
- Prepare the library for loading onto the flow cell.
VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001) contents
| Name | Acronym | Number of vials | Fill volume per vial (µl) |
|---|---|---|---|
| LunaScript RT SuperMix | LS RT | 1 | 10 |
| Q5 HS Master Mix | Q5 | 1 | 35 |
| VX Midnight Primer A | VMP A | 1 | 15 |
| VX Midnight Primer B | VMP B | 1 | 15 |
| VX BC Frag Mix 01-12 | VXB01-VXB12 | 12 | 10 |
| VX AMPure XP Beads | VXP | 1 | 45 |
| VX Rapid Adapter F | VRA F | 1 | 12 |
| VX Elution Buffer | VELB | 1 | 50 |
| VX Running Buffer | VRB | 1 | 250 |
| Negative Control | NEG | 1 | 10 |
| Flush Buffer | FB | 3 | 1170 |
| Flush Tether | FLT | 1 | 200 |
VolTRAX PCR Tiling 1-12 COVID-19 Kit barcode sequences
| Component | Sequence |
|---|---|
| VXB01 | AAGAAAGTTGTCGGTGTCTTTGTG |
| VXB02 | TCGATTCCGTTTGTAGTCGTCTGT |
| VXB03 | GAGTCTTGTGTCCCAGTTACCAGG |
| VXB04 | TTCGGATTCTATCGTGTTTCCCTA |
| VXB05 | CTTGTCCAGGGTTTGTGTAACCTT |
| VXB06 | TTCTCGCAAAGGCAGAAAGTAGTC |
| VXB07 | GTGTTACCGTGGGAATGAATCCTT |
| VXB08 | TTCAGGGAACAAACCAAGTTACGT |
| VXB09 | AACTAGGCACAGCGAGTCTTGGTT |
| VXB10 | AAGCGTTGAAACCTTTGTCCTCTC |
| VXB11 | GTTTCATCTATCGGAGGGAATGGA |
| VXB12 | CAGGTAGAAAGAAGCAGAATCGGA |
VSK-PTC001 chemistry
The VolTRAX PCR Tiling 1-12 COVID-19 Kit generates overlapping amplicons in a tiled fashion across the SARS-CoV-2 genome. First, the RNA samples are reverse-transcribed and then amplified by tiled PCR using separate primer pools. The primer pools are combined and SPRI purified before moving into the rapid barcoding stage which attaches Rapid Barcodes to the DNA ends for samples to be pooled.
14. Run a VolTRAX PCR Tiling 1-12 COVID-19 Kit
Materials
- Input RNA in 10 mM Tris-HCl, pH 8.0
- VolTRAX PCR Tiling 1-12 COVID-19 Kit (VSK-PTC001)
- VolTRAX Cartridge Pack (VCT-V2002B)
Consumables
- 0.2 ml thin-walled PCR tubes
- Qubit™ dsDNA HS Assay Kit (ThermoFisher, Q32851)
Equipment
- VolTRAX V2b
- P20 pipette and tips
- P10 pipette and tips
Optional equipment
- Qubit™ fluorometer (or equivalent for QC check)
VolTRAX V2b pipette tip compatibility
We have found that certain pipette tips give better results during the reagent loading and library extraction. Using the wrong tip type can lead to sample loss. The Mettler Toledo (Rainin™) UNV 10 µl tips have been verified as having the correct geometry for loading reagents on the VolTRAX cartridge. We recommend the Mettler Toledo (Rainin™) UNV 20 µl tips for extracting the prepared library at the end of the run.
Board test
The Board test should be carried out on your VolTRAX device before proceeding with PCR experiments. The most recent models of the VolTRAX V2b feature improved temperature calibration for PCR, and the Board test checks whether you have a version of the device that is PCR-compatible.
Start the VolTRAX software. You will be presented with the following options for protocols to run. Select "Board Test" to start the protocol and follow the instructions in the software.

Board test results
If the board test has been successful, you can proceed with PCR experiments.

If the board test has failed, please contact Support.

Click "Continue", which will take you back to the protocol selection screen. Select "VolTRAX PCR Tiling 1-12 COVID-19" to start the protocol and follow the instructions in the software.

Protocol preview
Once an option is selected from the menu, the UI will show a preview of the steps involved in library preparation. The example below is for the Configuration Kit.

When prompted by the UI, connect your VolTRAX device to the computer. When it is connected, you will then be prompted to insert a cartridge into the device.
Prime the cartridge as instructed in the UI by twisting the Priming Fluid container anti-clockwise and removing the foil tab to allow the fluid to enter the cartridge.
Select Continue when each step is completed.
Please refer to the videos displayed in the VolTRAX UI, which demonstrate how to perform each step in the protocol.
Loading solutions into the VolTRAX V2b cartridge
The VolTRAX V2b device can measure solution volumes loaded into the cartridge. The GUI displays a coloured-pixel grid to show:
- Blue: Volume of solution too low. You will need to load more solution to proceed with the protocol.
- Green: Correct volume of solution.
- Orange: slightly overfilled. You will still be able to proceed with the protocol.
- Red: solution overfilled. You will need to cancel the experiment and start again.

Wait for the library prep to finish.
A timer can be found on the right hand side of the UI, indicating the remaining time.
Cancelling a run
To cancel a run, select Cancel in the top right corner of the GUI.
The user is able to look through all the steps of the selected protocol before confirming cancellation.
The run will stop immediately and return the user to the "Start VolTRAX" page. Another run can be started immediately.
Note: The cartridge must be replaced if reagents have been input during a cancelled run. If no reagent has been inserted, the cartridge can be used again.
At the end of the experiment, extract the prepared library from the E port of the VolTRAX cartridge. Dispense the library into a 0.2 ml PCR tube for preparation of the sequencing mix, as discussed in the following section.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Run history
The UI provides a run history option to view previously-run protocols, which can be accessed from the link in the top right corner.

Clicking Open on any of the protocols will navigate the user to the relevant data folder in Windows File Explorer:

15. Priming and loading the SpotON flow cell
Materials
- Flush Buffer (FB)
- Flush Tether (FLT)
Consumables
- MinION Flow Cell
- 1.5 ml Eppendorf DNA LoBind tubes
Equipment
- P1000 pipette and tips
- P100 pipette and tips
Open the MinION Mk1B lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check instructions in the MinKNOW protocol for more information.
Slide the priming port cover clockwise to open the priming port.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles (a few µls).
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 ul, to draw back 20-30 ul, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.

To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the VX Running Buffer (VRB) tube by pipetting up and down.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| VX Running Buffer (VRB) | 64 µl |
| DNA library | 11 µl |
| Total | 75 µl |
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the priming port and close the lid of the sequencing device.
You are ready to begin your sequencing experiment.
Please refer to the MinKNOW protocol for instructions on setting up your sequencing experiment.
16. Overview of the VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001)
The VSK-VPS001 RNA library preparation kit
The VolTRAX RT-PCR Sequencing Kit 1-12 generates PCR amplicons from the user's input RNA using the VolTRAX V2b platform to improve consistency in library preparation due to the precise fluid handling of the device.
This kit provides the reagents required to generate reverse-transcribed and PCR-amplified material from RNA samples using VolTRAX automation to prepare the library. The user provides their own PCR primers to generate custom amplicons, before barcoding and pooling multiple samples together for sequencing. We recommend using 10 mM Tris-HCl pH 8.0 as a buffer for the primers, and using the VolTRAX device to optimise the PCR conditions. When loading PCR primers onto the VolTRAX, we recommend loading at 3X the desired reaction concentration.
Below is an overview of the library preparation stages using a VolTRAX V2b, blue cartridges and the VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001).
Overview of the VolTRAX RT-PCR Sequencing Kit 1-12 protocol
1. Loading a protocol
- Use the protocol selector in the UI to load the VolTRAX RT-PCR Sequencing Kit 1-12 protocol.
- When prompted, connect your VolTRAX V2b device, press Continue and await connection.
2. System QC - Cartridge Test
- When prompted, connect a cartridge to the VolTRAX device and press Continue to proceed to the Cartridge Test.
3. Loading the Priming Fluid and reagents into the cartridge
- Follow the instructions on the equipment required and pipetting technique.
- Prime the cartridge by twisting the oil container and removing the foil tab.
- Load the appropriate reagents, custom primers and extracted RNA into the ports as instructed by the UI.
- You will be given the option to adjust the PCR timings and temperatures to allow you to use custom primers.
- The reagents will be manipulated by the VolTRAX V2b to prepare the library for nanopore sequencing.
4. Extracting the library from the cartridge
- Follow the extraction instructions, using UI as a visual guide, to slowly extract the library from the cartridge, then transfer it into an Eppendorf tube.
- Prepare the library for loading onto the flow cell.
VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001) contents
| Name | Acronym | Cap colour | Number of vials | Fill volume per vial (µl) |
|---|---|---|---|---|
| LunaScript RT SuperMix | LS RT | Blue | 1 | 10 |
| Q5 HS Master Mix | Q5 | Orange | 1 | 35 |
| VX BC Frag Mix | VXB01-12 | Amber | 12 | 10 |
| VX AMPure XP Beads | VXP | Pink | 1 | 45 |
| VX Rapid Adapter F | VRA F | Green | 1 | 12 |
| VX Elution Buffer | VELB | Black | 1 | 50 |
| VX Running Buffer | VRB | Red | 1 | 250 |
| Negative Control | NEG | Clear | 1 | 10 |
| Flush Buffer | FB | Blue | 3 | 1170 |
| Flush Tether | FLT | White cap, purple label | 1 | 200 |
VolTRAX RT-PCR Sequencing Kit 1-12 barcode sequences
| Component | Sequence |
|---|---|
| VXB01 | AAGAAAGTTGTCGGTGTCTTTGTG |
| VXB02 | TCGATTCCGTTTGTAGTCGTCTGT |
| VXB03 | GAGTCTTGTGTCCCAGTTACCAGG |
| VXB04 | TTCGGATTCTATCGTGTTTCCCTA |
| VXB05 | CTTGTCCAGGGTTTGTGTAACCTT |
| VXB06 | TTCTCGCAAAGGCAGAAAGTAGTC |
| VXB07 | GTGTTACCGTGGGAATGAATCCTT |
| VXB08 | TTCAGGGAACAAACCAAGTTACGT |
| VXB09 | AACTAGGCACAGCGAGTCTTGGTT |
| VXB10 | AAGCGTTGAAACCTTTGTCCTCTC |
| VXB11 | GTTTCATCTATCGGAGGGAATGGA |
| VXB12 | CAGGTAGAAAGAAGCAGAATCGGA |
VSK-VPS001 chemistry
The VolTRAX RT-PCR Sequencing Kit 1-12 generates amplicons across target regions or organisms of interest within the extracted RNA sample. First, the RNA samples are reverse-transcribed and then amplified by PCR using the user-defined primers. The samples are purified with AMPure XP beads before tagmenting the DNA amplicons with the Rapid Barcodes, adding the sequencing adapter and pooling for sequencing.
17. Run a VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001)
Materials
- Input RNA in 10 mM Tris-HCl, pH 8.0
- VolTRAX RT-PCR Sequencing Kit 1-12 (VSK-VPS001)
- PCR primers in 10 mM Tris-HCl, pH 8.0
- VolTRAX Cartridge Pack (VCT-V2002B)
Consumables
- 0.2 ml thin-walled PCR tubes
- Qubit™ dsDNA HS Assay Kit (ThermoFisher, Q32851)
Equipment
- VolTRAX V2b
- P20 pipette and tips
- P10 pipette and tips
Optional equipment
- Qubit™ fluorometer (or equivalent for QC check)
VolTRAX V2b pipette tip compatibility
We have found that certain pipette tips give better results during the reagent loading and library extraction. Using the wrong tip type can lead to sample loss. The Mettler Toledo (Rainin™) UNV 10 µl tips have been verified as having the correct geometry for loading reagents on the VolTRAX cartridge. We recommend the Mettler Toledo (Rainin™) UNV 20 µl tips for extracting the prepared library at the end of the run.
Board test
The Board test should be carried out on your VolTRAX device before proceeding with PCR experiments. The most recent models of the VolTRAX V2b feature improved temperature calibration for PCR, and the Board test checks whether you have a version of the device that is PCR-compatible.
Start the VolTRAX software. You will be presented with the following options for protocols to run. Select "Board Test" to start the protocol and follow the instructions in the software.

Board test results
If the board test has been successful, you can proceed with PCR experiments.

If the board test has failed, please contact Support.

Click "Continue", which will take you back to the protocol selection screen. Select "VolTRAX RT-PCR Sequencing Kit" to start the protocol and follow the instructions in the software.

Protocol preview
Once an option is selected from the menu, the UI will show a preview of the steps involved in library preparation. The example below is for the Configuration Kit.

When prompted by the UI, connect your VolTRAX device to the computer. When it is connected, you will then be prompted to insert a cartridge into the device.
Prime the cartridge as instructed in the UI by twisting the Priming Fluid container anti-clockwise and removing the foil tab to allow the fluid to enter the cartridge.
Select Continue when each step is completed.
Please refer to the videos displayed in the VolTRAX UI, which demonstrate how to perform each step in the protocol.
Loading solutions into the VolTRAX V2b cartridge
The VolTRAX V2b device can measure solution volumes loaded into the cartridge. The GUI displays a coloured-pixel grid to show:
- Blue: Volume of solution too low. You will need to load more solution to proceed with the protocol.
- Green: Correct volume of solution.
- Orange: slightly overfilled. You will still be able to proceed with the protocol.
- Red: solution overfilled. You will need to cancel the experiment and start again.

When loading PCR primers onto the VolTRAX, we recommend loading at 3X the desired reaction concentration.
Adjust the PCR parameters
The PCR parameters form is always populated with the default parameters, however you can adjust the times and temperatures if you are using custom primers. This form enables 3-step PCR by default, however the parameters may be modified to allow 2-step PCR as described below.

Cycle Count
This section allows you to adjust the number of PCR cycles for your application and incorporate touchdown PCR cycles. Touchdown PCR is an approach that can increase the specificity of a PCR by reducing the amount of off-target priming. The annealing step begins at a temperature several degrees higher than the Tm of the primers. Over the following cycles, the annealing temperature is gradually reduced until it is several degrees below the Tm of the primers.
- Number of Touchdown Cycles: allows control over the number of cycles where touchdown is applied. These cycles are separate from the "Number of Cycles" field below.
- Number of Cycles (Post-Touchdown): total number of PCR cycles to complete after the touchdown PCR cycles.
Total Number of Cycles = Number of Touchdown Cycles + Number of Cycles
Denaturation
- Time (seconds): allows control over the duration of the denaturation step of the PCR.
- First Cycle Extra Time (seconds): this parameter gives you additional control over the initial denaturation step. If required, extend the time for the first denaturation step. If an extended first denaturation step is not required, leave the value at 0.
- Temperature (°C): allows you to set the temperature (°C) for the denaturation step of the PCR.
- Minimum Temperature (°C): this is the temperature at which the countdown for the denaturation step of the cycle will start. It is recommended that this is set to be 0.5°C less than the denaturation temperature.
Annealing
- Time (seconds): allows you to control the duration of the annealing step of the PCR.
- Temperature (°C): allows you to set the temperature (°C) for the annealing step of the PCR. This temperature is the annealing temperature at the start of the touchdown process.
- Maximum Temperature (°C): this is the temperature at which the countdown for the extension step of the cycle will start. It is recommended that this is set to be 0.5°C more than the annealing temperature.
- Total Temperature Change Over Touchdown Cycles (°C): allows to modify the extension temperature over the touchdown PCR cycles (e.g. a value of -5 with 5 touchdown cycles would reduce the annealing temperature of the reaction by 1°C on each touchdown PCR cycle. The subsequent cycles would use the Temperature -5).
Extension:
- Time (seconds): allows you to control the duration of the extension step of the PCR.
- Final Cycle Extra Time (seconds): this parameter gives additional control over the final extension step of the PCR. If required, extend the time for the final extension step. If an extended final extension step is not required, leave the value at 0.
- Temperature (°C): allows you to set the temperature (°C) for the extension steps of the PCR. This temperature is the annealing and extension temperature at the start of the touchdown process.
- Minimum Temperature (°C): this is the temperature at which the countdown for the extension step of the cycle will start. It is recommended that this is set to be 0.5°C less than the extension temperature.
To set up 2-step PCR: Set all Extension step fields to 0. Please note that this will result in no extra time being applied to the final cycle's last step. If extra time is required in the final cycle's last step, make the following adjustments:
- Fill in the desired values for the Cycle Count, Denaturation, and Annealing fields (the annealing step in this case is both annealing and extension).
- Set the Extension Time field to 0 (the extension will complete immediately once the minimum temperature is reached).
- Set the Final Cycle Extra Time field to the desired additional time on the final Annealing step.
- Set the Temperature field to the temperature of the final annealing step, taking the touchdown gain into account.
- Set the Minimum Temperature field to 0°C (this will immediately trigger the step timer).
Wait for the library prep to finish.
A timer can be found on the right hand side of the UI, indicating the remaining time.
Cancelling a run
To cancel a run, select Cancel in the top right corner of the GUI.
The user is able to look through all the steps of the selected protocol before confirming cancellation.
The run will stop immediately and return the user to the "Start VolTRAX" page. Another run can be started immediately.
Note: The cartridge must be replaced if reagents have been input during a cancelled run. If no reagent has been inserted, the cartridge can be used again.
At the end of the experiment, extract the prepared library from the E port of the VolTRAX cartridge. Dispense the library into a 0.2 ml PCR tube for preparation of the sequencing mix, as discussed in the following section.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Run history
The UI provides a run history option to view previously-run protocols, which can be accessed from the link in the top right corner.

Clicking Open on any of the protocols will navigate the user to the relevant data folder in Windows File Explorer:

18. Priming and loading the SpotON flow cell
Materials
- Flush Buffer (FB)
- Flush Tether (FLT)
Consumables
- MinION Flow Cell
- 1.5 ml Eppendorf DNA LoBind tubes
Equipment
- P1000 pipette and tips
- P100 pipette and tips
Open the MinION Mk1B lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check instructions in the MinKNOW protocol for more information.
Slide the priming port cover clockwise to open the priming port.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles (a few µls).
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 ul, to draw back 20-30 ul, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.

To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the VX Running Buffer (VRB) tube by pipetting up and down.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| VX Running Buffer (VRB) | 64 µl |
| DNA library | 11 µl |
| Total | 75 µl |
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the priming port and close the lid of the sequencing device.
You are ready to begin your sequencing experiment.
Please refer to the MinKNOW protocol for instructions on setting up your sequencing experiment.






