cDNA-PCR 条形码测序试剂盒 V14 (SQK-PCB114.24) (PCB_9201_v114_revG_11Feb2026)
MinION: Protocol
cDNA-PCR 条形码测序试剂盒 V14 (SQK-PCB114.24) V PCB_9201_v114_revG_11Feb2026
本文档介绍了一种最便捷高效的全长 cDNA 测序流程。
该流程具有以下特点:
- 实现最高测序产量
- 产量优于传统的 cDNA 合成方法
- 可检测剪接变异及融合转录本
- 支持多达24个不同样本的混合测序
- 仅兼容 R10.4.1 测序芯片
仅供研究使用
FOR RESEARCH USE ONLY
概览
本文档介绍了一种最便捷高效的全长 cDNA 测序流程。
该流程具有以下特点:
- 实现最高测序产量
- 产量优于传统的 cDNA 合成方法
- 可检测剪接变异及融合转录本
- 支持多达24个不同样本的混合测序
- 仅兼容 R10.4.1 测序芯片
仅供研究使用
1. 实验方案概览
本品为早期试用产品
如需有关早期试用计划的更多信息,请参阅本文了解产品的不同发布阶段。
请确保您始终使用本方案的最新版本。
cDNA-PCR 条形码测序试剂盒-24 V14 实验指南简介
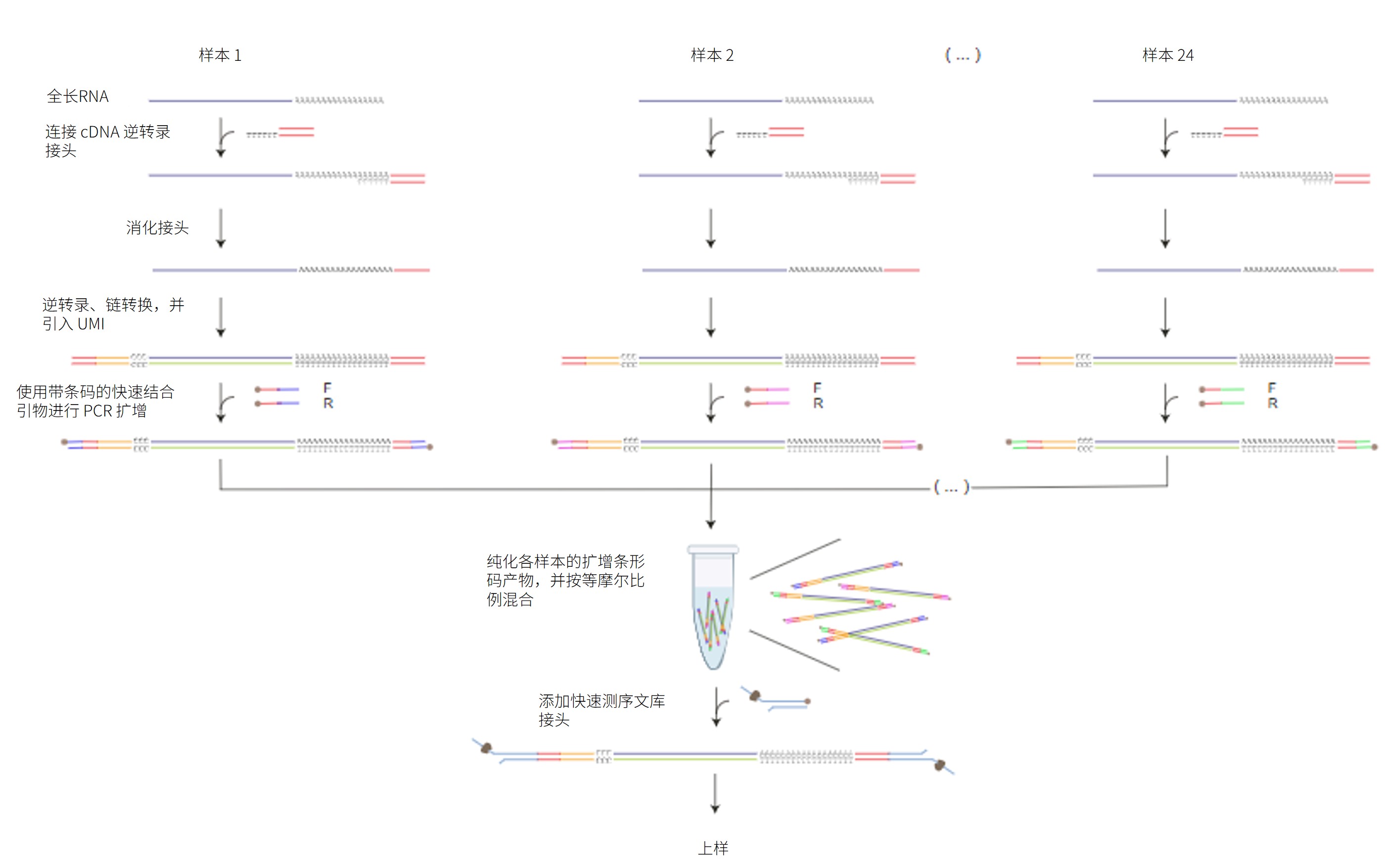
本方案详述了如何采用链转换法及 cDNA-PCR 条形码测序试剂盒-24 V14(SQK-PCB114.24)对多个 cDNA 样本进行测序。该试剂盒包含 24 种不同的条形码,可在单次测序中实现最多 24 个样本的混样测序。
实验采用链转换方法富集全长转录本,并在此过程中引入唯一分子标识(UMI)。随后,使用带有 5' 标签的引物对双链 cDNA 进行 PCR 扩增,并同步引入样本特异性条形码。扩增后的带条码产物经混合后加入快速测序文库接头,构建测序文库。
为帮助用户熟悉操作流程,或排查文库制备过程中可能出现的问题,建议使用 RNA 对照样本(RCS,源自 RNA 参照品扩展包 EXP-RCS001)首先进行对照实验。
测序工作流程:
实验准备
您将需要:
- 提取RNA,并根据起始DNA/RNA质控实验指南评估其长度、浓度和纯度。 质量评估步骤对确保实验成功至关重要。
- 确保您已准备好测序试剂盒、正确的仪器以及第三方试剂
- 下载数据收集和分析软件
- 检查您的测序芯片上有足够的活性纳米孔,以确保测序良好运行
文库制备
下表概述了文库制备所需的步骤,包括时间安排和可以中止的节点。
| 文库制备 | 步骤 | 时间 | 中止节点 |
|---|---|---|---|
| 逆转录及链转换 | 以 Poly(A)+ RNA(或总RNA)为模板合成全长cDNA,并引入唯一分子标识符(UMI) | 170 分钟 | -20°C 过夜 |
| PCR 筛选全长转录本 | 使用带有不同条形码的快速结合引物对 cDNA 进行 PCR 扩增 | 40 分钟 | 若为短期保存或重复使用(例如在清洗芯片后再次上样),我们建议将文库置于4℃保存。 若为单次使用长期保存,我们建议将文库置于-80℃。 |
| 快速测序文库接头连接 | 将测序接头连接至PCR产物 | 5 分钟 | 我们强烈建议您在接头连接完成后尽快开始测序。 |
| 测序芯片预处理及上样 | 对测序芯片进行预处理,然后将 cDNA 文库加至芯片中进行测序。 | 10 分钟 |
测序和分析
您将需要:
- 使用 MinKNOW 软件开始测序。该软件会通过测序仪收集原始数据,并将其识别成碱基序列。
- 非必需: 使用EPI2ME软件,选择所需工作流程进行进一步分析。
实验方案适用性
本实验方案只适用于与以下产品搭配使用:
- cDNA-PCR 条形码测序试剂盒-24 V14 (SQK-PCB114.24)
- R10.4.1 测序芯片(FLO-MIN114)
- 测序芯片冲洗试剂盒(EXP-WSH004)
- RNA 参照品扩展包(EXP-RCS001)
- 快速测序文库接头辅助扩展包 V14(EXP-RAA114)
- 测序辅助扩展包 V14 (EXP-AUX003)
- 测序芯片预处理试剂盒 V14 (EXP-FLP004)
- MinION Mk1D - MinION Mk1D IT 配置要求
2. 实验器材及耗材
材料
- 10 ng 富集 RNA(带 PolyA 尾或去除核糖体 RNA),或 500 ng 总 RNA
- cDNA-PCR 条形码测序试剂盒 V14 (SQK-PCB114.24)
耗材
- MinION/GridION测序芯片
- NEBNext®快速连接反应缓冲液(NEB,B6058)
- 2M U/ml 的 T4 DNA连接酶 (NEB,M0202)
- RNaseOUT™, 40 U/μl(Life Technologies, 10777019)
- Lambda 核酸外切酶(NEB,M0262L)
- Thermolabile 核酸外切酶 I(NEB, M0568)
- USER (尿嘧啶-特异性切除试剂) 酶(NEB, M5505L)
- 10mM 脱氧核糖核苷三磷酸(dNTP)溶液(例如 NEB N0447)
- Maxima H Minus 逆转录酶 (200 U/µL)与 5X RT 缓冲液一起提供(ThermoFisher, EP0752)
- LongAmp 热启动 Taq 酶 2X 预混液(NEB, M0533)
- Agencourt RNAClean XP 磁珠(Beckman Coulter™,A63987)
- Agencourt AMPure XP 磁珠(Beckman Coulter™,A63881)
- 牛血清白蛋白(BSA)(50 mg/mL)(例如 Invitrogen™ UltraPure™ BSA 50 mg/mL, AM2616)
- Qubit™ dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(ThermoFisher,Q32851)
- Qubit™ RNA HS Assay(RNA高灵敏度检测)试剂盒(ThermoFisher,Q32852)
- 无核酸酶水(如ThermoFisher,AM9937)
- 新制备的70%乙醇(用无核酸酶水配制)
- 1.5 ml Eppendorf DNA LoBind 离心管
- Qubit™ 分析管(Invitrogen, Q32856)
- 0.2 ml 薄壁PCR管
仪器
- MinION 或 GridION 测序仪
- MinION 及GridION 测序芯片遮光片
- Hula混匀仪(低速旋转式混匀仪)
- 适用于1.5ml Eppendorf 离心管的磁力架
- 迷你离心机
- 微孔板离心机
- 涡旋混匀仪
- 热循环仪
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
- 盛有冰的冰桶
- 计时器
- Qubit™ 荧光计(或用于质控检测的等效仪器)
- Agilent 生物分析仪(或等效仪器)
根据本实验指南,每个样本需准备 10 ng 富集 RNA(带 PolyA 尾或去除核糖体 RNA),或 500 ng 总 RNA。
第三方试剂
Oxford Nanopore Technologies 推荐您使用本实验指南中列出的所有第三方试剂,并已对其进行验证。我们尚未对其它替代试剂进行测试。
我们建议您按制造商说明准备待用的第三方试剂。
测序芯片质检
我们强烈建议您在开始测序实验前,对测序芯片的活性纳米孔数进行质检。质检需在您收到 MinION/GridION/PremethION 测序芯片12周内进行。Oxford Nanopore Technologies会对活性孔数量少于以下标准的芯片进行替换*:请您按照测序芯片质检文档中的说明进行芯片质检。
| 测序芯片 | 芯片上的活性孔数确保不少于 |
|---|---|
| MinION/GridION 测序芯片 | 800 |
| PromethION 测序芯片 | 5000 |
*(请注意:自收到之日起,芯片须一直贮存于Oxford Nanopore Technologies推荐的条件下。且质检结果须在质检后的两天内递交给我们。)
cDNA-PCR 条形码测序试剂盒-24 V14 (SQK-PCB114.24)内容物
我们正在将试剂盒中的条形码引物包装更改为96孔板形式。同时,孔板形式的引物浓度已由原瓶装形式的 10 µM 降低至 1 µM。这一改动将减少塑料浪费,并支持自动化应用。
请务必根据试剂盒的包装形式和条形码引物的浓度,严格按照相应说明进行操作。
条形码引物以 1 µM 浓度分装于孔板中
| 名称 | 缩写 | 管盖颜色 | 管数 | 每管溶液体积 (μl) |
|---|---|---|---|---|
| 模板链转换引物 II | SSPII | 蓝紫色 | 1 | 350 |
| 逆转录引物 | RTP | 黄色 | 1 | 200 |
| cDNA 逆转录接头 | CRTA | 琥珀色 | 1 | 200 |
| 退火缓冲液 | AB | 橙色 | 1 | 200 |
| 快速测序文库接头 | RA | 绿色 | 1 | 15 |
| 接头缓冲液 | ADB | 透明 | 1 | 100 |
| 洗脱缓冲液 | EB | 黑色 | 2 | 500 |
| 短片段缓冲液 | SFB | 透明 | 3 | 13,000 |
| 测序缓冲液 | SB | 红色 | 1 | 700 |
| 文库颗粒 | LIB | 粉色 | 1 | 600 |
| 文库溶液 | LIS | 白色管盖,粉色标签 | 1 | 600 |
| 测序芯片系绳 | FCT | 紫色 | 1 | 200 |
| 测序芯片冲洗液 | FCF | 透明管盖,浅蓝色标签 | 1 | 15,500 |
| 浓度为 1 µM 的条形码引物 1-24 | - | 两板,每板三套条形码引物组合 | 每孔 12µl |
条形码引物以 10 µM 浓度分装于管装
| 名称 | 缩写 | 管盖颜色 | 管数 | 每管溶液体积 (μl) |
|---|---|---|---|---|
| 模板链转换引物 II | SSPII | 蓝紫色 | 1 | 350 |
| 逆转录引物 | RTP | 黄色 | 1 | 200 |
| cDNA 逆转录接头 | CRTA | 琥珀色 | 1 | 200 |
| 退火缓冲液 | AB | 橙色 | 1 | 200 |
| 快速测序文库接头 | RA | 绿色 | 1 | 15 |
| 接头缓冲液 | ADB | 透明 | 1 | 100 |
| 洗脱缓冲液 | EB | 黑色 | 2 | 500 |
| 短片段缓冲液 | SFB | 透明 | 4 | 7,500 |
| 测序缓冲液 | SB | 红色 | 1 | 700 |
| 文库颗粒 | LIB | 粉色 | 1 | 600 |
| 文库溶液 | LIS | 白色管盖,粉色标签 | 1 | 600 |
| 条形码引物 1-24 | BP01-24 | 白色 | 24 (每种条形码引物各1管) | 10 |
| 测序芯片系绳 | FCT | 紫色 | 1 | 200 |
| 测序芯片冲洗液 | FCF | 透明管盖,浅蓝色标签 | 1 | 8,000 |
3. 逆转录与链转换
材料
- 10 ng 富集 RNA(带 PolyA 尾或去除核糖体 RNA),或 500 ng 总 RNA
- cDNA 逆转录接头(CRTA)
- 退火缓冲液(AB)
- 短片段缓冲液(SFB)
- 逆转录引物(RTP)
- 链转换引物 II(SSPII)
耗材
- NEBNext®快速连接反应缓冲液(NEB,B6058)
- 2M U/ml 的 T4 DNA连接酶 (NEB,M0202)
- Lambda 核酸外切酶(NEB,M0262L)
- USER (尿嘧啶-特异性切除试剂) 酶(NEB, M5505L)
- Agencourt RNAClean XP 磁珠(Beckman Coulter™,A63987)
- 10 mM dNTP 溶液(如 NEB,N0447)
- Maxima H Minus 逆转录酶 (200 U/µL)与 5X RT 缓冲液一起提供(ThermoFisher, EP0752)
- RNaseOUT™, 40 U/μl(Life Technologies, 10777019)
- Qubit™ RNA HS Assay(RNA高灵敏度检测)试剂盒(ThermoFisher,Q32852)
- 无核酸酶水(如ThermoFisher,AM9937)
- 1.5 ml Eppendorf DNA LoBind 离心管
- Qubit™ 分析管(Invitrogen, Q32856)
- 0.2 ml 薄壁PCR管
仪器
- 迷你离心机
- 热循环仪
- Qubit™ 荧光计(或用于质控检测的等效仪器)
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
测序芯片质检
我们建议您在开始文库制备之前,对测序芯片的活性纳米孔数量进行质检,以确保其足够支持实验的顺利进行。
详情请参阅 MinKNOW 实验指南中的测序芯片质检说明 。
操作 RNA 样本前的实验室准备
为取得最佳实验结果,我们建议您在操作 RNA 之前,对实验空间和设备进行充分准备,以最大程度减少 RNA 酶和其他污染物的影响:
- 使用 RNaseZap 和无尘擦拭纸清洁操作台表面。
- 使用 RNaseZap 和无尘擦拭纸清洁所有设备,如移液器、离心管架、离心机和涡旋混匀仪等。
- 使用全新的移液枪头和新鲜配制的试剂,以降低污染风险。
请将以下试剂解冻,并使用迷你离心机短暂离心后,按照下表说明充分混匀。随后将试剂置于冰上保存备用。
| 试剂 | 1.于室温下解冻 | 2.瞬时离心 | 3.吹打混匀 |
|---|---|---|---|
| cDNA 逆转录接头(CRTA) | ✓ | ✓ | ✓ |
| 退火缓冲液(AB) | ✓ | ✓ | ✓ |
| 短片段缓冲液(SFB) | ✓ | ✓ | ✓ |
| 逆转录引物(RTP) | ✓ | ✓ | ✓ |
| 模板链转换引物 II(SSPII) | ✓ | ✓ | ✓ |
| NEBNext® 快速连接反应缓冲液 | ✓ | ✓ | 涡旋振荡混匀 |
| T4 DNA 连接酶 2M U/ml | 非冰冻状态 | ✓ | ✓ |
| RNaseOUT | 非冰冻状态 | ✓ | ✓ |
| Lambda 核酸外切酶 | 非冰冻状态 | ✓ | ✓ |
| USER(尿嘧啶-特异性切除试剂)酶 | 非冰冻状态 | ✓ | ✓ |
| 10 mM dNTP 溶液 | ✓ | ✓ | ✓ |
| Maxima H Minus 逆转录酶 | 非冰冻状态 | ✓ | ✓ |
| Maxima H Minus 5x RT 缓冲液 | ✓ | ✓ | 涡旋振荡混匀 |
请务必涡旋振荡 NEBNext 快速连接反应缓冲液,以充分混匀。
查看是否有残留沉淀。涡旋振荡至少30秒以溶解所有沉淀。
如需进行对照实验,请将起始样本替换为 RNA 参照品扩展包(EXP-RCS001)中稀释后的 10 μl RNA 对照样本(RCS)。
本步骤会因试剂盒中 RNA 对照样本(RCS)的浓度不同而略有差异。请务必根据您所使用的 RNA 对照样本(RCS)浓度选择正确的操作方法。
我们已将新版 EXP-RCS001中 RNA 对照样本(RCS)管装浓度由 15 ng/μl 提升至 50 ng/μl。
| 批次:RCS001.10.xxxx 及之前 | 批次:RCS001.20.0001 及以后 |
|---|---|
| 较低浓度的 RNA 对照样本(RCS): 15 ng/µl | 较高浓度的 RNA 对照样本(RCS): 50 ng/µl |
- 将 RNA 参照品(RCS)于室温下解冻,瞬时离心后用移液枪吹打混匀。
- 在 1.5 ml 的 Eppendorf DNA LoBind 管中按如下比例稀释RNA 参照品(RCS):
对高浓度 RNA 对照样本(RCS)——试剂盒批次在 RCS001.20.0001 或以上:
| 试剂 | 体积 |
|---|---|
| RNA 参照品(RCS) | 1 μl |
| 无核酸酶水 | 46 μl |
| 总体积 | 47 μl |
注意: 该稀释液的配制量适用于 4 个样本,请根据实际需要按比例调整制备体积。
- 吹打混匀10–20次,瞬时离心。
- 使用 10 μl 稀释后的 RNA 对照样本(RCS)作为起始 RNA。
对低浓度 RNA 对照样本(RCS)——试剂盒批次在 RCS001.10.xxxx 或以下:
| 试剂 | 体积 |
|---|---|
| RNA 参照品(RCS) | 1 μl |
| 无核酸酶水 | 14 μl |
| 总体积 | 15 μl |
注意: 该稀释液的配制量适用于 1 个样本,请根据实际需要按比例调整制备体积。
- 吹打混匀10–20次,瞬时离心。
- 使用 10 μl 稀释后的 RNA 对照样本(RCS)作为起始 RNA。
使用无核酸酶水配制各 RNA 样本
- 将 10 ng 带Poly(A)尾的 RNA 或 500 ng 总 RNA 转移至一 0.2 ml 薄壁 PCR 管中;
- 如不足 10 μl,请加入无核酸酶水补足;
- 为避免不必要的打断,请轻弹试管以充分混匀;
- 使用迷你离心机瞬时离心。
在 0.2 ml PCR 管内,分别为每个样本按下表配制反应体系:
| 试剂 | 体积 |
|---|---|
| RNA | 10 µl |
| cDNA 逆转录接头(CRTA) | 1 µl |
| 退火缓冲液(AB) | 1 µl |
| 总体积 | 12 µl |
CRTA 为一段具有 poly(T) 突出端的双链接头,可与 RNA 分子 poly(A) 尾的末端碱基特异性结合, 从而确保 RNA 实现全长逆转录,并可准确评估 poly(A) 尾的长度。退火缓冲液(AB) 用于增强 CRTA 与 RNA 的连接效率。
轻弹离心管以充分混合,并瞬时离心。
在热循环仪中 60°C 孵育 5分钟,随后室温放置 5分钟 冷却。
向上述含 RNA 样本的各0.2 ml PCR 管中加入以下试剂:
| 试剂 | 体积 |
|---|---|
| 上一步处理后的 RNA 样本 | 12 µl |
| NEBNext® 快速连接反应缓冲液 | 3.6 µl |
| T4 DNA 连接酶 2M U/ml | 1.4 µl |
| RNaseOUT | 1 µl |
| 总体积 (含所有试剂) | 18 µl |
吹打10次充分混匀,瞬时离心。
注意: 请轻柔混匀,避免产生气泡。
室温下孵育10分钟。
向上述各0.2 ml PCR 管中加入以下试剂:
| 试剂 | 体积 |
|---|---|
| 上一步处理后的 RNA 样本 | 18 µl |
| Lambda 核酸外切酶 | 1 µl |
| USER (尿嘧啶-特异性切除试剂) 酶 | 1 µl |
| 总体积 (含所有试剂) | 20 µl |
Lambda 外切酶和 USER 酶(尿嘧啶特异性切除试剂)为第三方试剂,用于处理已连接的 CRTA 接头,以为逆转录步骤做好准备。Lambda 外切酶和 USER 酶可降解已连接 CRTA 结构中的下链,使逆转录引物(RTP)能够与上链的 CRTA 序列结合,从而为 RNA 的逆转录反应提供启动位点。
轻弹 PCR 管充分混匀各试剂后,瞬时离心;
在 37°C 条件下于热循环仪中孵育 5 分钟。
将各样品分别转至一支干净的 1.5 ml Eppendorf DNA LoBind 离心管中。
涡旋振荡以重悬 RNAClean XP 磁珠。
向各反应体系中分别加入 36 µl 重悬的 RNAClean XP 磁珠,轻弹离心管以充分混合。
将离心管置于Hula混匀仪(低速旋转式混匀仪)上室温孵育5分钟。
将各样品瞬时离心后置于磁力架上,待磁珠与液相完全分离。保持离心管在磁力架上不动,用移液枪吸去清液。
保持离心管在磁力架上不动,以 100µl 的短片段缓冲液(SFB)按以下步骤洗涤各管内磁珠:
1.使用 100µl 短片段缓冲液(SFB)洗涤磁珠。
2.保持磁力架在实验台上不动,将离心管旋转180°。
3.待磁珠向磁铁处移动并聚集后,再将离心管旋转180°(即离心管回到原位),并再次静置至磁珠与液相分离。
4.注意避免扰动磁珠,用移液枪小心吸除短片段缓冲液(SFB),并弃去。
重复上述步骤。
将离心管瞬时离心后置于磁力架上。用移液枪吸走残留的缓冲液。将磁珠在空气中干燥约 30 秒,避免干至表面开裂。
将离心管从磁力架上移开。将各管磁珠分别重悬于12 µl 无核酸酶水中。
室温下孵育10分钟。
将离心管置于磁力架上,直到磁珠和液相分离,且洗脱液澄清无色。
将每个样本的12 µl 洗脱液分别转移至一支新的 0.2ml 薄壁 PCR 管中。
向上述各0.2 ml PCR 管中加入以下试剂:
| 试剂 | 体积 |
|---|---|
| 上一步洗脱所得样本 | 12 µl |
| 逆转录引物(RTP) | 1 µl |
| 10 mM dNTP 溶液 | 1 µl |
| 总体积 (含所有试剂) | 14 µl |
RT Primer (RTP) is a single stranded primer and binds downstream of the poly(A) tail of the RNA transcript to prime for reverse transcription.
轻弹 PCR 管充分混匀各试剂后,瞬时离心;
室温下孵育5分钟。
向上述各0.2 ml PCR 管中加入以下试剂:
| 试剂 | 体积 |
|---|---|
| 上一步中添加了逆转录引物的样本 | 14 µl |
| Maxima H Minus 5X RT 缓冲液 | 4.5 µl |
| RNaseOUT | 1 µl |
| 模板链转换引物 II(SSPII) | 2 µl |
| 总体积 (含所有试剂) | 21.5 µl |
链转换引物 II(SSPII)可与第一条已合成 cDNA 链 5' 端的脱氧胞苷(dC)配对结合,引导逆转录酶启动链转换,并继续合成第二条 cDNA 链。
轻弹离心管以充分混合,并瞬时离心。
在热循环仪中以 42°C 孵育两分钟。
向每管中加入 1 μl Maxima H Minus 逆转录酶。每管反应总体积应为 22.5 μl。
轻弹离心管以充分混合,并瞬时离心。
在热循环仪中依照以下程序孵育样本:
| 步骤 | 温度 | 时间 | 循环数 |
|---|---|---|---|
| 逆转录及链转换 | 42°C | 30 分钟 | 1 |
| 热灭活 | 85°C | 5 分钟 | 1 |
| 温度保持 | 4°C | ∞ |
样本可直接用于下一步骤。如有需要,您也可在此阶段将样本置于 −20°C 条件下过夜保存。
4. 通过 PCR 筛选全长转录本
材料
- 条形码引物 (BP01–24)
- 洗脱缓冲液(EB)
耗材
- LongAmp 热启动 Taq 酶 2X 预混液(NEB, M0533)
- Thermolabile 核酸外切酶 I(NEB, M0568)
- Agencourt AMPure XP 磁珠(Beckman Coulter™,A63881)
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
- 新制备的70%乙醇(用无核酸酶水配制)
- 无核酸酶水(如ThermoFisher,AM9937)
- 1.5 ml Eppendorf DNA LoBind 离心管
- Qubit™ 分析管(Invitrogen, Q32856)
- 0.2 ml PCR 管
仪器
- 迷你离心机
- 微孔板离心机
- 热循环仪
- 涡旋混匀仪
- Hula混匀仪(低速旋转式混匀仪)
- 适用于1.5ml Eppendorf 离心管的磁力架
- 盛有冰的冰桶
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
- Qubit™ 荧光计(或用于质控检测的等效仪器)
- Agilent 生物分析仪(或等效仪器)
本试剂盒支持多达 24 个样本的混样测序。默认情况下,每个样本仅需进行一次 25 µl 的 PCR 反应。当仅需对 2–3 个样本进行混样测序时,为获得足量产物,建议每个样本进行两次独立的 PCR 扩增;如仅处理 1 个样本,则应参考 PCR-cDNA 测序试剂盒 V14(SQK-PCS114)操作指南,进行四次独立的 PCR 扩增。上述建议旨在确保获得足量的 PCR 产物,以优化测序芯片的使用表现。
逆转录酶会抑制 PCR 反应,因此逆转录产物必须经过适当稀释,方可进行 PCR 扩增。
注意: 请为每个样本分别使用一组唯一的条形码引物。
请将以下试剂解冻,并使用离心机短暂离心后,按照下表说明充分混匀。随后将试剂置于冰上保存备用。
| 试剂 | 1.于室温下解冻 | 2.瞬时离心 | 3.吹打混匀 |
|---|---|---|---|
| 条形码引物(BP01 - BP24) | ✓ | ✓ | ✓ |
| 洗脱缓冲液(EB) | ✓ | ✓ | ✓ |
| LongAmp® 热启动 Taq酶 2X 预混液 | ✓ | ✓ | ✓ |
| Thermolabile 核酸外切酶 I | 非冰冻状态 | ✓ | ✓ |
条形码板中的各孔仅限一次性使用。使用前请确认所选孔密封完好;一旦刺穿或开启,不得再次使用。
将经过逆转录的 RNA 样本瞬时离心。
为每个样本分别准备一支 0.2 ml PCR 管,并在每管中加入 5 μl 逆转录产物。
PCR 扩增仅需使用 5 μl 逆转录产物,请勿将全部 22.5 μl 的逆转录反应体系用于单次 PCR 反应。
请确保根据所使用的 SQK-PCB114.24 试剂盒的包装形式选择正确的操作方法。
除试剂盒包装形式的变更外,我们还将条形码引物的浓度从管装形式的 10 µM 降低至孔板形式的 1 µM,以支持自动化应用,并优化试剂盒的整体使用体验。
请注意:因此变更,不同包装形式的试剂盒在 PCR 建库步骤中所需的各组分体积将有所不同,请根据实际使用的试剂盒包装形式选择对应的反应配方。
在每支含有逆转录产物的 0.2 ml PCR 管中,于室温按下表配置反应体系:
孔板式包装(条形码引物浓度为 1 µM)
| 试剂 | 体积 |
|---|---|
| 上一步逆转录反应所得样本 | 5 μl |
| 唯一条形码引物(BP01–24) | 7.5 μl |
| LongAmp® 热启动 Taq 酶 2X 预混液 | 12.5 μl |
| 总体积 (含所有试剂) | 25 μl |
管板式包装(条形码引物浓度为 10 µM)
| 试剂 | 体积 |
|---|---|
| 上一步逆转录反应所得样本 | 5 μl |
| 唯一条形码引物(BP01–24) | 0.75 μl |
| 无核酸酶水 | 6.75 μl |
| LongAmp® 热启动 Taq 酶 2X 预混液 | 12.5 μl |
| 总体积 (含所有试剂) | 25 μl |
轻柔吹打混匀。
按下表条件进行扩增:
| 步骤 | 温度 | 时间 | 循环数 |
|---|---|---|---|
| 预变性 | 95°C | 30 秒 | 1 |
| 变性 | 95°C | 15 秒 | 10-18* |
| 退火 | 62°C | 15 秒 | 10-18* |
| 延伸 | 65°C | 每 kb 60 秒 | 10-18* |
| 最后延伸 | 65°C | 6 分钟 | 1 |
| 温度保持 | 4°C | ∞ |
**我们建议初始设定为 14 个 PCR 循环,后续可根据实验需求在推荐范围内进行调整。
如需了解更多信息,请参阅文档:PCR-cDNA 测序试剂盒中 PCR 循环数变化的影响。
向每个 PCR 反应管中直接加入 1 μl Thermolabile 核酸外切酶 I。轻弹离心管以充分混合,并瞬时离心。
添加 Thermolabile 外切酶 I 的目的是去除未能成功退火的多余引物。
将离心管置于热循环仪中,依次在 37°C 孵育 5 分钟,随后在 80°C 孵育两分钟。
将各样品分别转至一支干净的 1.5 ml Eppendorf DNA LoBind 离心管中。
涡旋振荡以重悬AMPure XP磁珠。
向每支 1.5 ml Eppendorf DNA LoBind 管中加入 18 µl 重悬后的 AMPure XP 磁珠。
将离心管置于Hula混匀仪(低速旋转式混匀仪)上室温孵育5分钟。
准备5 ml新制备的70%乙醇(用无核酸酶水配制)。
将样品瞬时离心后置于磁力架上,待磁珠与液相完全分离。保持离心管在磁力架上不动,用移液枪吸去清液。
保持试管在磁力架上不动,以 100µl 新鲜制备的70%乙醇洗涤磁珠。小心不要碰到磁珠。用移液枪将乙醇吸走并弃掉。
重复上述步骤。
将离心管瞬时离心后置于磁力架上。用移液枪吸走残留的乙醇。将磁珠在空气中干燥约 30 秒,避免干至表面开裂。
将离心管从磁力架上移开。将各管磁珠分别重悬于 12 µl 洗脱缓冲液中(EB)。
室温下孵育10分钟。
将离心管置于磁力架上,直到磁珠和液相分离,且洗脱液澄清无色。
将每个样本的 12 µl 洗脱产物分别转移至新的 1.5 ml Eppendorf DNA LoBind 管中。
- 将含有 cDNA 文库 的洗脱液转移至新的 1.5 ml Eppendorf DNA LoBind 管中
- 将磁珠丢弃
对于每个样本,取 1 μl 扩增后的 cDNA,使用 Qubit 荧光计和 Agilent 生物分析仪(或等效仪器)检测片段长度、浓度和质量,进行文库质控(QC)。
部分样本在凝胶电泳中可能呈现出明显的高分子量条带,而非预期的弥散性片段分布,此类文库通常与较差的测序结果相关联。我们发现,适当减少 PCR 循环数并重新扩增,可改善上述问题。
将扩增后带有条形码的 cDNA 样本按等摩尔比例混合,合计 50 fmol,并用洗脱缓冲液(EB)补足至最终体积 11 μl。
| 质量 | 片段长度为 0.5 kb 时的摩尔浓度 | 当片段长度为 1.5 kb 时的摩尔浓度 | 当片段长度为 3 kb 时的摩尔浓度 |
|---|---|---|---|
| 5 ng | 16 fmol | 5 fmol | 3 fmol |
| 10 ng | 32 fmol | 11 fmol | 5 fmol |
| 15 ng | 49 fmol | 16 fmol | 8 fmol |
| 20 ng | 65 fmol | 22 fmol | 11 fmol |
| 25 ng | 81 fmol | 27 fmol | 13 fmol |
| 50 ng | 154 fmol | 51 fmol | 26 fmol |
| 100 ng | 324 fmol | 108 fmol | 54 fmol |
如扩增后的 cDNA 总量超过 50 fmol,剩余部分可冷冻保存,用于今后的测序实验,届时文库构建可直接从“接头连接”步骤开始。建议避免反复冻融,以防 DNA 降解影响文库质量。
文库保存建议
若为 短期 保存或重复使用(例如在清洗芯片后再次上样),我们建议将文库置于Eppendorf LoBind 离心管中 4℃ 保存。 若为一次性使用且储存时长 超过3个月 ,我们建议将文库置于Eppendorf LoBind 离心管中 -80℃ 保存。
5. 接头连接
材料
- 快速测序文库接头(RA)
- 接头缓冲液(ADB)
- 洗脱缓冲液(EB)
耗材
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- 迷你离心机
- 盛有冰的冰桶
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
本试剂盒及其实验指南中使用的快速测序文库接头(RA)不可与其他测序接头互换使用。
将下表所列试剂置于室温解冻,经迷你离心机瞬时离心后吹打混匀:
| 试剂 | 1.于室温下解冻 | 2.瞬时离心 | 3.吹打混匀 |
|---|---|---|---|
| 快速测序文库接头(RA) | 非冰冻状态 | ✓ | ✓ |
| 接头缓冲液(ADB) | 非冰冻状态 | ✓ | ✓ |
在一支新的 1.5 ml Eppendorf DNA LoBind 管中,按以下比例稀释快速测序文库接头(RA),并吹打混匀:
| 试剂 | 体积 |
|---|---|
| 快速测序文库接头(RA) | 1.5 μl |
| 接头缓冲液(ADB) | 3.5 μl |
| 总体积 | 5 μl |
向扩增后的 cDNA 文库中加入 1 μl 稀释后的快速测序接头(RA),使最终体积达到 12 μl。
轻弹离心管以充分混合,并瞬时离心。
室温下孵育5分钟。
瞬时离心。
构建好的文库即可用于测序芯片上样。在上样前,请将文库置于冰上保存。
6. MinION 及 GridION 测序芯片的预处理及上样
材料
- 测序芯片冲洗液(FCF)
- 测序芯片系绳(FCT)
- 文库溶液(LIS)
- 文库颗粒(LIB)
- 测序缓冲液(SB)
耗材
- MinION/GridION测序芯片
- 牛血清白蛋白(BSA)(50 mg/mL)(例如 Invitrogen™ UltraPure™ BSA 50 mg/mL, AM2616)
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- MinION 或 GridION 测序仪
- MinION 及GridION 测序芯片遮光片
- P1000 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
请注意:本试剂盒仅兼容 R10.4.1 测序芯片(FLO-MIN114)。
从冰箱中取出测序芯片,在室温下放置 20 分钟,以便在预处理和上样时更清晰地观察到传感器阵列。
测序芯片的预处理及上样
我们建议所有新用户在首次运行测序芯片前,观看视频测序芯片的预处理及上样。
使用文库溶液
对大多数测序实验,我们建议用户使用文库颗粒(LIB)为测序芯片上样。但对于黏稠度较高的文库,借助文库颗粒进行上样可能较为困难,建议使用文库缓冲液(LIS)替代。
于室温下解冻测序缓冲液(SB)、文库颗粒(LIB)或文库溶液(LIS)、测序芯片系绳(FCT)和测序芯片冲洗液(FCF)。完全解冻后,涡旋振荡混匀。然后瞬时离心,置于冰上。
为在 MinION R10.4.1 测序芯片(FLO-MIN114)上获得最优测序表现并提高测序产出,请向测序芯片预处理液中加入终浓度为 0.2 mg/ml 的牛血清白蛋白(BSA)。
注意: 我们不建议使用其他类型的白蛋白(如重组人血清白蛋白)。
配制含 BSA 的芯片预处理液:将下表试剂加入至 1.5 ml 的 Eppendorf 管中,在室温下颠倒并吹打混匀。
| 试剂 | 体积(每张芯片) |
|---|---|
| 测序芯片冲洗液 (FCF) | 1170 µl |
| 浓度为 50 mg/mL 的牛血清白蛋白 (BSA) | 5 µl |
| 测序芯片系绳(FCT) | 30 µl |
| 总体积 | 1205 µl |
打开 MinION 或 GridION 测序仪的盖子,将测序芯片插入金属固定夹的下方。用力向下按压预处理孔孔盖处,以确保正确的热、电接触。


为文库上样前,完成测序芯片质检,查看可用孔数目。
如此前已对测序芯片进行过质检,则此步骤可省略。
详情请参阅 MinKNOW 实验指南中的测序芯片质检说明 。
顺时针转动测序芯片的预处理孔孔盖,使预处理孔显露出来。

小心地从测序芯片中反旋吸出缓冲液。请勿吸出超过 20-30 µl的缓冲液,并确保芯片上的纳米孔阵列一直有缓冲液覆盖。将气泡引入阵列会对纳米孔造成不可逆转地损害。
将预处理孔打开后,检查孔周围是否有小气泡。请按照以下方法,从孔中排出少量液体以清除气泡:
1.将 P1000 移液枪转至 200µl 刻度。
2.将枪头垂直插入预处理孔中。
3.反向转动移液枪量程调节转纽,直至移液枪刻度在 220-230 µl之间,或直至您看到有少量缓冲液进入移液枪枪头。
注意: 肉眼检查,确保从预处理孔到传感器阵列的缓冲液连续且无气泡。

通过预处理孔向芯片中加入 800µl 预处理液,避免引入气泡。等待5分钟。在此期间,请按照以下步骤准备用于上样的DNA文库。

将含有文库颗粒的LIB管用移液枪吹打混匀。
LIB管内的文库颗粒分散于悬浮液中。由于颗粒沉降速度非常快,因此请在混匀颗粒后立即使用。
对于大多数测序实验,我们建议您使用文库颗粒(LIB)。但如文库较为粘稠,您可考虑使用文库溶液(LIS)。
在一支新的1.5ml Eppendorf DNA LoBind离心管内,将所有试剂按以下顺序混合:
| 试剂 | 每张测序芯片的上样体积 |
|---|---|
| 测序缓冲液(SB) | 37.5 µl |
| 文库颗粒 (LIB),临用前混匀;或文库溶液 (LIS) | 25.5 µl |
| DNA 文库 | 12 µl |
| 总体积 | 75 µl |
完成测序芯片的预处理:
- 轻轻地翻起SpotON上样孔盖,使SpotON上样孔显露出来。

- 通过预处理孔(而 非 SpotON加样孔)向芯片中加入200µl预处理液,避免引入气泡。

临上样前,用移液枪轻轻吹打混匀制备好的文库。
通过 SpotON 加样孔向芯片中逐滴加入 75µl 制备好的文库。确保液滴流入孔内后,再加下一滴。

轻轻合上 SpotON 加样孔孔盖,确保塞头塞入加样孔内。合上预处理孔盖。


为获得最佳测序产出,在文库样本上样后,请立即在测序芯片上安装遮光片。
我们建议在清洗芯片并重新上样时,将遮光片保留在测序芯片上。一旦文库从测序芯片中吸出,即可取下遮光片。
按下述步骤安装测序芯片遮光片:
1.小心将遮光片的前沿(平端)与金属固定夹的边沿对齐。 注意: 请勿将遮光片强行压到固定夹下方。
2.将遮光片轻轻盖在测序芯片上。遮光片的 SpotON 加样孔孔盖缺口应与芯片上的 SpotON 加样孔孔盖接合,遮盖住整个测序芯片的前部。

MinION测序芯片的遮光片并非固定在测序芯片上,因此当为芯片加装遮光片后,请小心操作。
合上测序设备上盖,在 MinKNOW 上设置测序实验。
将测序芯片插入 MinION Mk1D 测序仪后,仪器上盖会覆盖于芯片上方,芯片四周可能留有一条小缝隙。此为正常现象,不影响设备性能。
请参阅此 常见问题解答 ,了解有关测序仪上盖的更多信息。

7. 数据采集和碱基识别
如何开始测序
在完成测序芯片的加样后,您即可在MinKNOW中启动测序实验。MinKNOW 软件负责仪器控制、数据采集以及实时碱基识别。有关设置和使用 MinKNOW 的详细信息,请参阅MinKNOW 实验指南。
您可以通过多种方式使用并设置MinKNOW:
- 在直接或远程连接到测序设备的计算机上。
- 直接在 GridION 或 PromethION 24/48 测序设备上。
有关在测序设备上使用 MinKNOW 的更多信息,请参阅相应设备的用户手册:
在MinKNOW中启动测序:
1.在 "开始 "(Start)页面上,选择 开始测序 (Start Sequencing)。
2.输入实验详情:例如实验名称,测序芯片位置及样本ID。
3.在"试剂盒"页面上,选择 PCR cDNA 条形码测序试剂盒-24 (SQK-PCB114-24) 。
4.在“实验配置”(Run Configuration)页面设置测序与输出参数,或保持默认值。
请注意: 如果在设置实验参数时关闭了碱基识别,您可在实验结束后,在 MinKNOW 中运行线下碱基识别。详情请参阅MinKNOW实验指南。
5.单击 "参数确认" 页面上的 开始 启动测序。
测序后数据分析
当于MinKNOW上完成测序后,您可按照“测序芯片的重复利用及回收”一节中的说明重复使用或返还测序芯片。
完成测序和碱基识别后,即可进行数据分析。有关碱基识别和后续分析选项的详细信息,请参阅数据分析文档。
在下游分析部分,我们将概述更多用于数据分析的选项。
8. 测序芯片的重复利用及回收
材料
- 测序芯片清洗剂盒(EXP-WSH004)
完成测序实验后,如您希望再次使用测序芯片,请按照“测序芯片清洗试剂盒实验指南”进行操作,并将清洗后的芯片置于 +2 至 +8℃ 保存。
您可在纳米孔社区获取测序芯片清洗试剂盒实验指南。
或者,请按照回收程序将测序芯片返还至Oxford Nanopore。
您可在 此处找到回收测序芯片的说明。
如果您遇到问题或对测序实验有疑问,请参阅本实验指南在线版本中的“疑难解答指南”一节。
9. 下游分析
下游分析
您可以选择以下几个途径来进一步分析经过碱基识别的数据:
EPI2ME 工作流程
Oxford Nanopore Technologies 通过 EPI2ME 平台提供了一系列针对高阶数据分析的生物信息学教程和工作流程。上述资源汇总于纳米孔社区的EPI2ME 板块。该平台通过描述性文字、生物信息学代码和示例数据,具象化地展示出我们的研究和应用团队发布在 GitHub 上的工作流程。
科研分析工具
Oxford Nanopore Technologies 的研发部门开发了许多分析工具,您可在 Oxford Nanopore 的 GitHub 资料库中找到。这些工具面向有一定经验的用户,并包含如何安装和运行软件的说明。工具是以源代码的形式提供的,因此我们仅提供有限的技术支持。
纳米孔社区用户开发的分析工具
如上述资源未能提供满足您研究需求的数据分析方法,请前往资源中心,查找适用的生物信息学工具。该工具库汇总了各种第三方用户开发、且在Github上开源的、针对纳米孔数据的生信分析工具。请注意,Oxford Nanopore Technologies不为这些工具提供支持,也不能保证它们与测序所用的最新的化学试剂/软件配置兼容。
10. DNA/RNA提取和文库制备过程中可能出现的问题
以下表格列出了提取和文库制备过程中的常见问题,以及可能的原因和解决方法。
我们还在 Nanopore 社区的Support板块提供了常见问题解答(FAQ)。
如果以下方案仍无法解决您的问题,请通过电邮(support@nanoporetech.com)或 纳米孔社区的在线支持(LiveChat)联系我们。
低质量样本
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 低纯度DNA(Nanodrop测定的DNA吸光度比值260/280<1.8,260/230 <2.0-2.2) | 用户所使用的DNA提取方法未能达到所需纯度 | 您可在 污染物专题技术文档中查看污染物对后续文库制备和测序实验的影响。请尝试其它不会导致污染物残留的 提取方法 。 请考虑将样品再次用磁珠纯化。 |
| RNA完整值低 (RNA完整值(RIN)<9.5,或 rRNA 在电泳凝胶上的条带呈弥散状) | RNA在提取过程中降解 | 请尝试其它RNA 提取方法.您可在NA完整值 文档中查看更多有关RNA完整值(RIN)的介绍。更多信息,请参阅 DNA/RNA 操作 页面。 |
| RNA的片段长度短于预期 | RNA在提取过程中降解 | 请尝试其它 RNA 提取方法。您可在RNA完整值 文档中查看更多有关RNA完整值(RIN)的介绍。更多信息,请参阅 DNA/RNA 操作 页面。 我们建议用户在无RNA酶污染的环境中操作,并确保实验设备没有受RNA酶污染. |
经AMPure磁珠纯化后的DNA回收率低
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 低回收率 | AMPure磁珠量与样品量的比例低于预期,导致DNA因未被捕获而丢失 | 1.AMPure 磁珠沉降速度较快,因此在将磁珠加入样品前,请务必充分重悬混匀。 2.当AMPure磁珠量与样品量的比值低于0.4:1时,所有的DNA片段都会在纯化过程中丢失。 |
| 低回收率 | DNA片段短于预期 | AMPure磁珠量与样品量的比值越低,针对短片段的筛选就越严格。每次实验时,请先使用琼脂糖凝胶(或其他凝胶电泳方法)确定起始DNA的长度,据此计算出合适的AMPure磁珠用量。 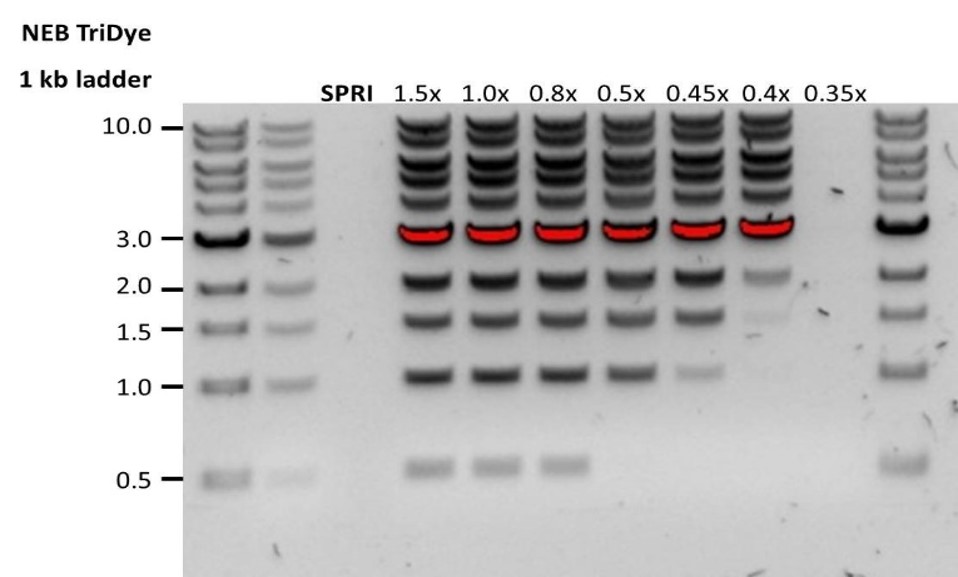 |
| 末端修复后的DNA回收率低 | 清洗步骤所用乙醇的浓度低于70% | 当乙醇浓度低于70%时,DNA会从磁珠上洗脱下来。请确保使用正确浓度的乙醇。请确保使用正确浓度的乙醇。 |
11. 测序过程中可能出现的问题
以下表格列出了提取和文库制备过程中的常见问题,以及可能的原因和解决方法。
我们还在 Nanopore 社区的Support板块提供了常见问题解答(FAQ)。
如果以下方案仍无法解决您的问题,请通过电邮(support@nanoporetech.com)或 纳米孔社区的在线支持(LiveChat)联系我们。
MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数 | 纳米孔阵列中引入了气泡 | 在对通过质控的芯片进行预处理之前,请务必排出预处理孔附近的气泡。否则,气泡会进入纳米孔阵列对其造成不可逆转地损害。视频中演示了避免引入气泡的最佳操作方法。 |
| MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数 | 测序芯片没有正确插入测序仪 | 停止测序,将芯片从测序仪中取出,再重新插入测序仪内。请确保测序芯片牢固嵌入测序仪中,并已到达目标温度。如用户使用的是GridION/PromethION测序仪,也可尝试将芯片插入仪器的其它芯片槽进行测序。 |
| MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数 | 文库中残留的污染物对纳米孔造成损害或堵塞 | 在测序芯片质检阶段,我们用芯片储存缓冲液中的质控DNA分子来评估活性纳米孔的数量。而在测序开始时,我们使用DNA文库本身来评估活性纳米孔的数量。因此,活性纳米孔的数量在这两次评估中会有约10%的浮动。如测序开始时报告的孔数明显降低,则可能是由于文库中的污染物对膜结构造成了损坏或将纳米孔堵塞。用户可能需要使用其它的DNA/RNA提取或纯化方法,以提高起始核酸的纯度。您可在 污染物专题技术文档中查看污染物对测序实验的影响。请尝试其它不会导致污染物残留的 提取方法。 |
MinKNOW脚本失败
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| MinKNOW显示 "Script failed”(脚本失败) | 重启计算机及MinKNOW。如问题仍未得到解决,请收集 MinKNOW日志文件并联系我们的技术支持。如您没有其他可用的测序设备,我们建议您先将装有文库的测序芯片置于4°C 储存,并联系我们的技术支持团队获取进一步储存上的建议。 |
纳米孔利用率低于40%
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 纳米孔利用率<40% | 测序芯片中的文库量不够 | 请确保您按照相应实验指南,向测序芯片中加入正确浓度和体积的测序文库。请在上样前对文库进行定量,并使用 Promega Biomath Calculator 等工具中的“dsDNA:µg to pmol”功能来计算DNA分子的摩尔量。 |
| 纳米孔利用率接近0 | 使用连接测序试剂盒,但接头并未与DNA成功连接 | 请确保您在“测序接头连接”步骤中使用的是NEBNext快速连接模块(E6056),以及SQK-LSK114试剂盒中的连接缓冲液(LNB)。同时,请确保每种试剂的用量正确。您可通过制备Lambda对照文库来检验第三方试剂的可用性。 |
| 纳米孔利用率接近0 | 使用连接测序试剂盒;但在接头连接后的纯化步骤中并未使用LFB 或SFB洗涤,而是使用了酒精 | 酒精可导致测序接头上的马达蛋白变性。请确保在测序接头连接后使用洗涤缓冲液(LFB或SFB)。 |
| 纳米孔利用率接近0 | 测序芯片中无系绳 | 系绳(FLT或FCT)随预处理液加至芯片。请确保在制备预处理液时,根据需求将 FLT 或 FCT 添加到相应的冲洗缓冲液 (FB) 或 测序芯片冲洗液 (FCF) 中。 |
读长短于预期
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 读长短于预期 | DNA样本降解 | 读长反映了起始DNA片段的长度。起始DNA在提取和文库制备过程中均有可能被打断。 1.请查阅纳米孔社区中的 提取方法 以获得最佳DNA提取方案. 2.在进行文库制备之前,请先跑电泳,查看起始DNA片段的长度分布。 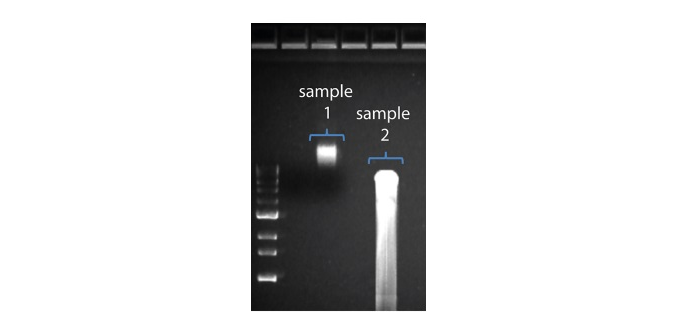 在上图中,样本1为高分子量DNA,而样本2为降解样本。 在上图中,样本1为高分子量DNA,而样本2为降解样本。3.在制备文库的过程中,请避免使用吹打或/和涡旋振荡的方式来混合试剂。轻弹或上下颠倒离心管即可。 |
大量纳米孔处于不可用状态
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
大量纳米孔处于不可用状态 (在通道面板和纳米孔活动状态图上以蓝色表示) 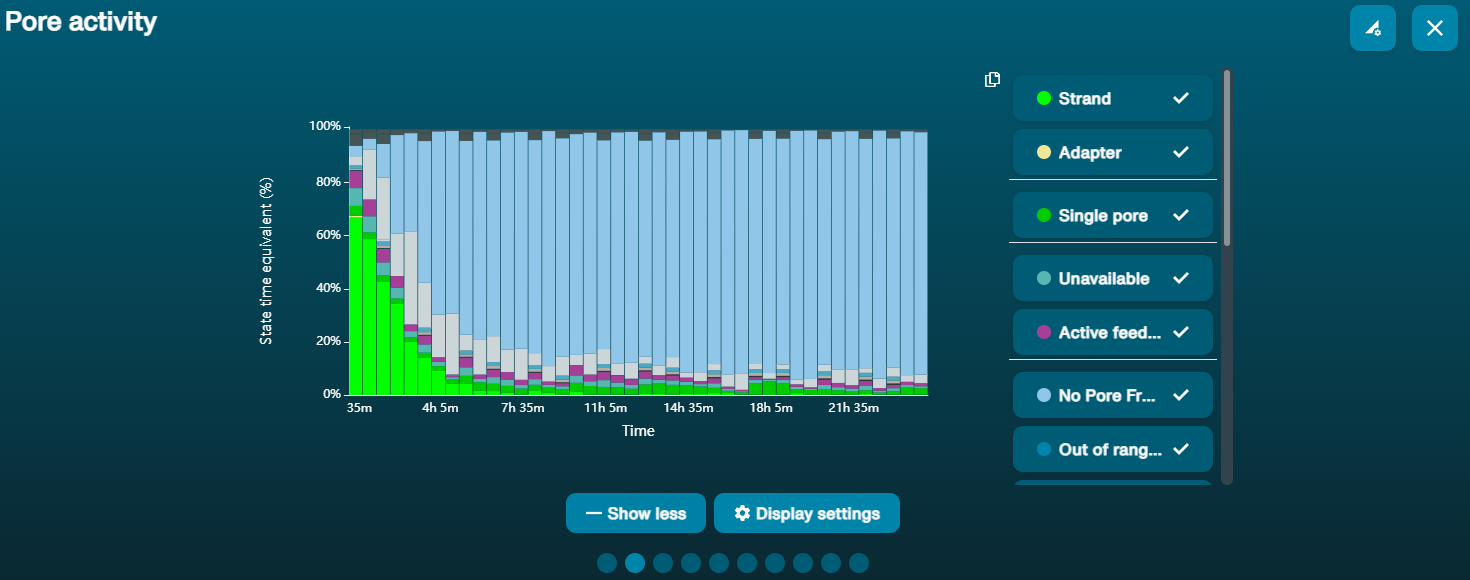 上方的纳米孔活动状态图显示:状态为不可用的纳米孔的比例随着测序进程而不断增加。 上方的纳米孔活动状态图显示:状态为不可用的纳米孔的比例随着测序进程而不断增加。 | 样本中含有污染物 | 使用MinKNOW中的“Unblocking”(疏通)功能,可对一些污染物进行清除。如疏通成功,纳米孔的状态会变为"测序孔"(sequencing pore)。若疏通后,状态为不可用的纳米孔的比例仍然很高甚至增加: 1.用户可使用 测序芯片冲洗试剂盒 (EXP-WSH004)进行核酸酶冲洗 操作,或 2.使用PCR扩增目标片段,以稀释可能导致问题的污染物。 |
大量纳米孔处于“失活”(Inactive)状态
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 大量纳米孔处于失活状态(在通道面板和纳米孔活动状态图上以浅蓝色表示。膜结构或纳米孔遭受不可逆转地损伤 | 测序芯片中引入了气泡 | 芯片预处理和文库上样过程中引入的气泡会对纳米孔带来不可逆转地损害。请观看 测序芯片的预处理及上样 视频了解最佳操作方法 |
| 大量纳米孔处于失活/不可用状态 | 存在与DNA共纯化的化合物 | 已知的化合物包括通常与植物基因组 DNA 相关的多糖等。 1.请参考植物叶片DNA提取方法. 2.使用QIAGEN PowerClean Pro试剂盒进行纯化。 3.利用QIAGEN REPLI-g试剂盒对原始gDNA样本进行全基因组扩增。 |
| 大量纳米孔处于失活/不可用状态 | 样本中含有污染物 | 您可在Contaminants 中查看污染物对测序实验的影响。请尝试其它不会导致污染物残留的提取方法。 |
温度波动
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 温度波动 | 测序芯片和仪器接触不良 | 检查芯片背面的金属板是否有热垫覆盖。重新插入测序芯片,用力向下按压,以确保芯片的连接器引脚与测序仪牢固接触。如问题仍未得到解决,请联系我们的技术支持。 |
未能达到目标温度
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| MinKNOW显示“未能达到目标温度” | 测序仪所处环境低于标准室温,或通风不良(以致芯片过热) | MinKNOW会限定测序芯片达到目标温度的时间。当超过限定时间后,系统会显示出错信息,但测序实验仍会继续。值得注意的是,在错误温度下测序可能会导致通量和数据质量(Q值)的降低。请调整测序仪的摆放位置,确保将其置于室温下、通风良好的环境中,再在MinKNOW中重启进程。有关 MinION 温度控制的更多信息,请点击 此链接查看。 |







