Ligation sequencing amplicons - custom PCR UMI (SQK-LSK109) (CPU_9107_v109_revH_09Oct2020)
MinION: Protocol
V CPU_9107_v109_revH_09Oct2020
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
- 1. Overview of the protocol
- 2. Primer design
- 3. Equipment and consumables
- 4. Computer requirements and software
Library preparation
- 5. UMI tagging
- 6. Early PCR and clean-up
- 7. Late PCR and clean-up
- 8. End-prep
- 9. Adapter ligation and clean-up
- 10. Priming and loading the SpotON flow cell
Sequencing and data analysis
Troubleshooting
1. Overview of the protocol
This is a Legacy product
This kit is available on the Legacy page of the store. We are in the process of gathering data to support the upgrade of this protocol to our latest chemistry. Further information regarding protocol upgrades will be provided on the Community as soon as they are available over the next few months. For further information on please see the product update page.
Features of this protocol:
This protocol is recommended for users who want to incorporate unique molecular identifiers (UMIs) into targeted amplicons.
Introduction to the protocol
This protocol may be used to incorporate unique molecular identifiers (UMIs) into amplicons to be sequenced using the Ligation Sequencing Kit (SQK-LSK109). Custom primers are used to target a specific locus for amplification and tag amplicons with UMIs. Subsequent rounds of universal amplification synthesise UMI amplicon copies which may then be sequenced. The resulting 1D reads are then clustered together based on their UMI identity to generate a single high accuracy read for each original UMI tagged molecule.
This protocol has been developed based on research by Oxford Nanopore Technologies and published literature: Søren M. Karst, Ryan M. Ziels, Rasmus H. Kirkegaard, Emil A. Sørensen, Daniel McDonald, Qiyun Zhu, Rob Knight and Mads Albertsen (2020) “Enabling high-accuracy long-read amplicon sequences using unique molecular identifiers with Nanopore or PacBio sequencing”. bioRxiv, 645903. doi: https://doi.org/10.1101/645903.
Steps in the sequencing workflow:
Prepare for your experiment
You will need to:
- Design and order primers as further explained in Primer Design of this document
- Extract your DNA and check its length, quantity and purity. The quality checks are performed during the protocol are essential in ensuring experimental success
- Ensure you have your sequencing kit, the correct equipment and third-party reagents
- Download the software for acquiring and analysing your data
- Check your flow cell to ensure it has enough pores for a good sequencing run
__Library preparation__
You will need to:
- Fragment and UMI tag the DNA
- Cycle 1: The top and bottom target strands are unilaterally tagged with a UMI tailed with a universal priming site
- Cycle 2: The opposite terminus is tagged to complete a dsDNA template bilaterally tagged with complimentary UMI sequences tailed with universal priming sites
- Amplify the DNA and prepare the DNA ends for adapter attachment
- The intermediate AMPure XP bead cleans between the early and late PCR steps reduce the presence of off-target amplification
- Attach sequencing adapters supplied in the kit to the DNA ends
- Prime the flow cell and load your DNA library into the flow cell
Sequencing and analysis
You will need to:
- Start a sequencing run using the MinKNOW software, which will collect raw data from the device and convert it into basecalled reads
Compatibility of this protocol
This protocol should only be used in combination with:
- Ligation Sequencing Kit (SQK-LSK109)
- R9.4.1 (FLO-MIN106) flow cells
- Flow Cell Wash kit (EXP-WSH004)
2. Primer design
Primer design
To carry out this custom PCR UMI amplification, you are required to design and order UMI tagging primers with gene specific primer (GSP) sequences incorporated. We strongly recommend the use of Primer3Plus to facilitate primer design for the given target. After identifying a target locus, we recommend adhering to the following parameters when designing custom primers:
- Target amplicon length of 130 — 1000 bp long
- Primer length of ~20 — 25 bp long
- Minimal secondary structure, self-complementary, or crosstalk
- Melting temperature of ~60°C, with no more than 3°C difference in melting temperature between the forward primer and reverse primer
Once a suitable primer pair has been identified, the sequence may be incorporated into the GSP UMI primer sequences below. Substitute the bold 3'N region with the corresponding orientation of GSP sequence.
Note: it is critical that the forward GSP sequence is added to the forward UMI primer sequence and vice versa; failure to do so will result in no target amplification.
| Primer name | Length | Primer sequences |
|---|---|---|
| GSP UMI fwd | 71 - 76 bp | GTATCGTGTAGAGACTGCGTAGGT TTVVVVTTVVVVTTVVVVTTVVVVTTT NNNNNNNNNNNNNNNNNNNN |
| GSP UMI rev | 70 - 75 bp | AGTGATCGAGTCAGTGCGAGTGTT TVVVVTTVVVVTTVVVVTTVVVVTTT NNNNNNNNNNNNNNNNNNNN |
| UVP fwd | 60 bp | GGTGCTGAAGAAAGTTGTCGG TGTCTTTGTGTTAACCGTATCGTG TAGAGACTGCGTAGG |
| UVP rev | 59 bp | GGTGCTGAAGAAAGTTGTCGG TGTCTTTGTGTTAACCAGTGATCG AGTCAGTGCGAGTG |
With the GSP sequence incorporated into the UMI primer sequence, proceed to order the custom UMI primers, as well as the UVP oligos in the table above. Synthesis of pure, full length oligos is essential, therefore users are advised to order the UMI oligos with PAGE purification, resuspended to 100 μM in TE buffer.
3. Equipment and consumables
Material
- 50,000-60,000 target strand copies of gDNA
- Flow Cell Priming Kit (EXP-FLP002)
- Ligation Sequencing Kit (SQK-LSK109)
- Custom GSP UMI primers
- UVP oligos
Consumibles
- 0.2 ml thin-walled PCR tubes
- 1.5 ml Eppendorf DNA LoBind tubes
- Tubo g-TUBE™ (Covaris, 520079)
- Microesferas Agencourt AMPure XP (Beckman Coulter™, A63881)
- Magnesium chloride (M1028)
- Módulo NEBNext® Ultra™ II End Repair/dA-Tailing (NEB, E7546)
- NEBNext Quick Ligation Module (NEB E6056)
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Platinum™ SuperFi™ II Green PCR Master Mix (ThermoFisher, cat # 12369010)
- Quick Calf Intestinal Phosphatase (NEB cat #M0525)
- Thermolabile Exonuclease I (NEB, M0568)
- Etanol al 70 % recién preparado en agua sin nucleasas
Instrumental
- Magnetic rack suitable for 0.2 ml PCR tubes
- Magnetic separation rack, suitable for 1.5 ml Eppendorf tubes
- Bioanalizador Agilent (o equivalente)
- P1000 pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
- Hula mixer (gentle rotator mixer)
- Thermal cycler
- Microfuge
Equipo opcional
- Qubit™ fluorometer (or equivalent for QC check)
For this protocol, we recommend starting with an input of 50,000-60,000 target strand copies.
Ligation Sequencing Kit contents (SQK-LSK109)
| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (µl) |
|---|---|---|---|---|
| DNA CS | DCS | Yellow | 1 | 50 |
| Adapter Mix | AMX | Green | 1 | 40 |
| Ligation Buffer | LNB | Clear | 1 | 200 |
| L Fragment Buffer | LFB | White cap, orange stripe on label | 2 | 1,800 |
| S Fragment Buffer | SFB | Grey | 2 | 1,800 |
| Sequencing Buffer | SQB | Red | 2 | 300 |
| Elution Buffer | EB | Black | 1 | 200 |
| Loading Beads | LB | Pink | 1 | 360 |
Flow Cell Priming Kit contents (EXP-FLP002)

| Name | Acronym | Cap colour | No. of vials | Fill volume per vial (μl) |
|---|---|---|---|---|
| Flush Buffer | FB | Blue | 6 | 1,170 |
| Flush Tether | FLT | Purple | 1 | 200 |
Calculating input requirements
The more target strand copies input into the PCR, the more UMI clusters can be expected. However, this will in turn result in decreased cluster density. Therefore, we recommend an input of 50,000 — 60,000 target strand copies as an ideal compromise of total clusters and cluster density.
Worked example for the human genome:
- The haploid human genome is approximately 3.235 Gbp long
- The average DNA basepair has an atomic mass of 660 g•mol-1, therefore the atomic mass of the haploid human genome is 3.235E+9 bp * 660 g•mol-1 = 2.135E+12 g•mol-1
- To find the mass of a single copy of the haploid human genome, divide the atomic mass by Avogadro's constant: 2.135E+12 g•mol-1 / 6.022E+23 mol-1 = 3.545E-12 g or 3.545 pg.
- With a mass of approximately 3.545 pg, the dsDNA of the haploid genome contains complimentary top and bottom strands; half this mass represents a single target strand copy, therefore: 3.545 pg / 2 = 1.773 pg per target strand copy.
- To find the input mass of gDNA required: 50,000 target strand copies * 1.773 pg = 88,650 pg or 88.650 ng.
- In this instance, an input mass of 100 ng human gDNA will represent approximately 57,803 target strand copies, satisfying the input requirements of 50,000-60,000 target strand copies whilst remaining easy to quantify.
Contaminants
This PCR method uses multiple cycles of universal amplification and there is a potential for the amplification of contaminants. We strongly recommend that users conduct the UMI tagging inside a clean PCR area to minimise contamination. For the PCR and clean-up step, we recommend users to carry out steps in a post-PCR area.
2X Platinum™ SuperFi™ II Green PCR Master Mix
We strongly recommend the use of 2X Platinum™ SuperFi™ II Green PCR Master Mix as we have found this high fidelity polymerase performs well for UMI PCRs. The green colour also helps control subsequent SPRI cleans without impacting PCR performance.
4. Computer requirements and software
MinION Mk1B IT requirements
Sequencing on a MinION Mk1B requires a high-spec computer or laptop to keep up with the rate of data acquisition. For more information, refer to the MinION Mk1B IT requirements document.
MinION Mk1C IT requirements
The MinION Mk1C contains fully-integrated compute and screen, removing the need for any accessories to generate and analyse nanopore data. For more information refer to the MinION Mk1C IT requirements document.
MinION Mk1D IT requirements
Sequencing on a MinION Mk1D requires a high-spec computer or laptop to keep up with the rate of data acquisition. For more information, refer to the MinION Mk1D IT requirements document.
Software for nanopore sequencing
MinKNOW
The MinKNOW software controls the nanopore sequencing device, collects sequencing data and basecalls in real time. You will be using MinKNOW for every sequencing experiment to sequence, basecall and demultiplex if your samples were barcoded.
For instructions on how to run the MinKNOW software, please refer to the MinKNOW protocol.
EPI2ME (optional)
The EPI2ME cloud-based platform performs further analysis of basecalled data, for example alignment to the Lambda genome, barcoding, or taxonomic classification. You will use the EPI2ME platform only if you would like further analysis of your data post-basecalling.
For instructions on how to create an EPI2ME account and install the EPI2ME Desktop Agent, please refer to this link.
Check your flow cell
We highly recommend that you check the number of pores in your flow cell prior to starting a sequencing experiment. This should be done within 12 weeks of purchasing for MinION/GridION/PromethION or within four weeks of purchasing Flongle Flow Cells. Oxford Nanopore Technologies will replace any flow cell with fewer than the number of pores in the table below, when the result is reported within two days of performing the flow cell check, and when the storage recommendations have been followed. To do the flow cell check, please follow the instructions in the Flow Cell Check document.
| Flow cell | Minimum number of active pores covered by warranty |
|---|---|
| Flongle Flow Cell | 50 |
| MinION/GridION Flow Cell | 800 |
| PromethION Flow Cell | 5000 |
5. UMI tagging
Material
- 50,000-60,000 target strand copies of gDNA
- Custom GSP UMI primers
Consumibles
- Platinum™ SuperFi™ II Green PCR Master Mix (ThermoFisher, cat # 12369010)
- 0.2 ml thin-walled PCR tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Thermolabile Exonuclease I (NEB, M0568)
- Quick Calf Intestinal Phosphatase (NEB cat #M0525)
Instrumental
- Microfuge
- Thermal cycler
DNA fragmentation
If the average fragment length of the sample exceeds 8 Kbp, this may make quantification difficult and lead to mis-quantified input target strand copies. To ensure accurate quantification is achieved, we recommend fragmenting DNA into ~8 kb lengths using a Covaris g-TUBE prior to sample quantification.
We recommend users conduct the UMI tagging inside a clean PCR area to minimise contamination.
Determine the required input mass of gDNA which yields 50,000-60,000 target strand copies.
Prepare the primers and 2X Platinum™ SuperFi™ II Green PCR Master Mix in accordance with manufacturer's instructions, and place on ice.
In a clean PCR area, set up the UMI tagging reaction in a 0.2 ml PCR tube as follows:
| Component | Volume |
|---|---|
| 2X Platinum™ SuperFi™ II Green PCR Master Mix | 7.5 µl |
| 10 µM custom GSP UMI fwd (500 nM final) | 0.75 µl |
| 10 µM custom GSP UMI rev (500 nM final) | 0.75 µl |
| gDNA | 50,000-60,000 cp |
| Nuclease-free water | up to 6 µl |
| Total | 15 µl |
Mix gently by flicking the tube and spin down.
Run in a thermal cycler using the following program:
| Step | Temperature | Ramp rate | Time | Cycles |
|---|---|---|---|---|
| Initial denaturation | 98°C | max | 3 min | 1 |
| Denaturation Annealing Extension | 98°C Touchdown from 66°C to 60°C 72°C | max 0.2°C/sec max | 30 sec 90 sec 90 sec | 2 |
| Final extension | 72°C | max | 5 min | 1 |
| Hold | 4°C | - | - | - |
In a clean 0.2 ml PCR tube, set up a reaction in the following order to remove the custom GSP UMI tagging primers:
| Component | Volume |
|---|---|
| UMI tagging reaction | 15 µl |
| Nuclease-free water | 1.5 µl |
| Thermolabile exonuclease I | 0.75 µl |
| Quick calf intestinal phosphatase | 0.75 µl |
| Total | 18 µl |
Mix gently by flicking the tube and spin down.
Using a thermal cycler, incubate at 37°C for 15 minutes and 80°C for 2 minutes.
Take forward 18 µl of the exonuclease treated UMI tagging reaction into the early PCR and clean-up step.
6. Early PCR and clean-up
Material
- UVP oligos
- Exonuclease-treated UMI tagging reaction
Consumibles
- 0.2 ml thin-walled PCR tubes
- Platinum™ SuperFi™ II Green PCR Master Mix (ThermoFisher, cat # 12369010)
- Magnesium chloride (M1028)
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- Microesferas Agencourt AMPure XP (Beckman Coulter™, A63881)
- Etanol al 70 % recién preparado en agua sin nucleasas
Instrumental
- Microfuge
- Thermal cycler
- Hula mixer (gentle rotator mixer)
- Magnetic rack suitable for 0.2 ml PCR tubes
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
- Vortex mixer
2X Platinum™ SuperFi™ II Green PCR Master Mix
We strongly recommend the use of 2X Platinum™ SuperFi™ II Green PCR Master Mix as we have found this high fidelity polymerase performs well for UMI PCRs. The green colour also helps control subsequent SPRI cleans without impacting PCR performance.
In a clean PCR area, prepare the universal amplification mastermix in a 0.2 ml PCR tube:
| Reagent | Volume |
|---|---|
| 2X Platinum™ SuperFi™ II Green PCR Master Mix | 55 µl |
| 50 mM MgCl2 (1 mM final) | 2.2 µl |
| 10 µM UVP fwd (100 nM final) | 1.1 µl |
| 10 µM UVP rev (100 nM final) | 1.1 µl |
| Nuclease-free water | 11 µl |
| Total | 70.4 µl |
Note: At 1X concentration the Platinum™ SuperFi™ II Green PCR Master Mix contains 1.5 mM MgCl2, this is further supplemented above to achieve an overall final concentration of 2.5 mM MgCl2.
Mix by pipetting and store on ice for further use.
Prepare the following reaction in a 0.2 ml PCR tube:
| Reagent | Volume |
|---|---|
| Exonuclease treated UMI tagging reaction | 18 µl |
| Universal amplification mastermix | 32 µl |
| Total | 50 µl |
Mix gently by flicking the tube and spin down.
Incubate using the following program:
| Step | Temperature | Ramp rate | Time | Cycles |
|---|---|---|---|---|
| Initial denaturation | 98°C | max | 3 min | 1 |
| Denaturation Annealing Extension | 98°C Touchdown from 70°C to 63°C 72°C | max 0.2°C/sec max | 20 sec 45 sec 90 sec | 5 |
| Denaturation Extension | 98°C 72°C | max max | 20 sec 2 min | 5 |
| Final extension | 72°C | max | 5 min | 1 |
| Hold | 4°C | - | - | - |
Note: An AMPure XP bead clean-up is carried out after 10 cycles to reduce off-target amplification, promoting target amplicons.
We recommend conducting the following steps in a post-PCR area.
Resuspend the AMPure XP beads by vortexing.
Add 45 µl of resuspended AMPure XP beads to the reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 10 minutes at room temperature.
Prepare 500 µl of fresh 70% ethanol in nuclease-free water.
Spin down the sample and pellet the beads on a magnet for 5 minutes. Keep the tube on the magnet until the eluate is clear, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 200 µl of freshly prepared 70% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 18 µl of nuclease-free water. Incubate for 5 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear.
Remove and retain 18 µl of eluate containing DNA library into a clean 0.2 ml thin-walled PCR tube.
Dispose of the pelleted beads.
Take forward the 18 µl of eluate containing DNA library into the 'Late PCR and Clean-up' step.
7. Late PCR and clean-up
Material
- DNA library eluate
- Universal amplification mastermix
Consumibles
- 0.2 ml thin-walled PCR tubes
- Microesferas Agencourt AMPure XP (Beckman Coulter™, A63881)
- Etanol al 70 % recién preparado en agua sin nucleasas
- 1.5 ml Eppendorf DNA LoBind tubes
Instrumental
- Microfuge
- Thermal cycler
- Hula mixer (gentle rotator mixer)
- Magnetic rack suitable for 0.2 ml PCR tubes
- Qubit™ fluorometer (or equivalent for QC check)
- P1000 pipette and tips
- P100 pipette and tips
- P200 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
- Vortex mixer
We recommend conducting the following steps in a post-PCR area.
In a clean 0.2 ml PCR tube, set up the following reaction:
| Reagent | Volume |
|---|---|
| DNA library eluate | 18 µl |
| Universal amplification mastermix | 32 µl |
| Total | 50 µl |
Mix gently by flicking the tube and spin down.
Incubate using the following program:
| Step | Temperature | Ramp rate | Time | Cycles |
|---|---|---|---|---|
| Initial denaturation | 98°C | max | 3 min | 1 |
| Denaturation Extension | 98°C 72°C | max max | 20 sec 2 min | 25 |
| Final extension | 72°C | max | 5 min | 1 |
| Hold | 4°C | max | - | - |
Note: if a yield of >200 fmols is not achieved, please optimise the amplification with additional cycles as required in future experiments.
Resuspend the AMPure XP beads by vortexing.
Add 45 µl of resuspended AMPure XP beads to the reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 10 minutes at room temperature.
Prepare 500 µl of fresh 70% ethanol in nuclease-free water.
Spin down the sample and pellet the beads on a magnet for 5 minutes. Keep the tube on the magnet until the eluate is clear, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 200 µl of freshly prepared 70% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 30 µl of nuclease-free water. Incubate for 5 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear.
Remove and retain 30 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads.
Quantify 1 µl of each eluted sample using a Qubit fluorometer.
Take the UMI tagged amplicons forwards to the end-prep step.
8. End-prep
Material
- 200 fmols of UMI tagged amplicons
Consumibles
- Módulo NEBNext® Ultra™ II End Repair/dA-Tailing (NEB, E7546)
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- 0.2 ml PCR tubes
- Microesferas Agencourt AMPure XP (Beckman Coulter™, A63881)
- Etanol al 70 % recién preparado en agua sin nucleasas
Instrumental
- Microfuge
- Thermal cycler
- Hula mixer (gentle rotator mixer)
- Magnetic rack suitable for 0.2 ml PCR tubes
- Qubit™ fluorometer (or equivalent for QC check)
- P1000 pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P2 pipette and tips
- Vortex mixer
We recommend analysing 1 µl of sample using the Agilent Bioanalyser to calculate 200 fmols.
Determine the average amplicon size from this data to calculate the input sample volume for the end-prep reaction.
Determine the volume of the cleaned-up PCR reaction that yields 200 fmols of UMI tagged amplicons.
Prepare the NEBNext Ultra II End Repair / dA-tailing Module reagents in accordance with manufacturer's instructions, and place on ice:
For optimal performance, NEB recommend the following:
Thaw all reagents on ice.
Ensure the reagents are well mixed.
Note: Do not vortex the Ultra II End Prep Enzyme Mix.Always spin down tubes before opening for the first time each day.
The NEBNext Ultra II End Prep Reaction Buffer may contain a white precipitate. If this occurs, allow the mixture(s) to come to room temperature and pipette the buffer several times to break up the precipitate, followed by a quick vortex to mix.
Do not vortex the NEBNext Ultra II End Prep Enzyme Mix.
It is important that the NEBNext Ultra II End Prep Reaction Buffer is mixed well by vortexing.
Check for any visible precipitate; vortexing for at least 30 seconds may be required to solubilise all precipitate.
In a 0.2 ml thin-walled PCR tube, combine the following:
| Reagent | Volume |
|---|---|
| UMI tagged amplicons | 200 fmols |
| Nuclease-free water | Up to 50 µl |
| Ultra II End-prep reaction buffer | 7 µl |
| Ultra II End-prep enzyme mix | 3 µl |
| Total | 60 µl |
Mix gently by flicking the tube and spin down.
Using a thermal cycler, incubate at 20°C for 10 minutes and 65°C for 10 minutes.
AMPure XP bead clean-up
It is recommended that the repaired/end-prepped DNA sample is subjected to the following clean-up with AMPure XP beads. This clean-up can be omitted for simplicity and to reduce library preparation time. However, it has been observed that omission of this clean-up can: reduce subsequent adapter ligation efficiency, increase the prevalence of chimeric reads, and lead to an increase in pores being unavailable for sequencing. If omitting the clean-up step, proceed to the next section (“Adapter Ligation and clean-up”).
Resuspend the AMPure XP beads by vortexing.
Add 60 µl of resuspended AMPure XP beads to the end-prep reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Prepare 500 µl of fresh 70% ethanol in nuclease-free water.
Spin down the sample and pellet on a magnet until the eluate is clear and colourless. Keep the plate on the magnetic rack, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 200 µl of freshly prepared 70% ethanol without disturbing the pellet. Wait for the beads to migrate towards the magnet and form a pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 63 µl nuclease-free water. Spin down and incubate for 5 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear.
Remove and retain 63 µl of eluate into a clean 1.5 ml Eppendorf DNA LoBind tube.
Quantify 1 µl of each eluted sample using a Qubit fluorometer.
Take forward the end-prepped DNA into the adapter ligation step.
9. Adapter ligation and clean-up
Material
- Adapter Mix (AMX)
- Ligation Buffer (LNB)
- Short Fragment Buffer (SFB)
- Elution Buffer (EB)
Consumibles
- NEBNext Quick Ligation Module (NEB E6056)
- 1.5 ml Eppendorf DNA LoBind tubes
- Microesferas Agencourt AMPure XP (Beckman Coulter™, A63881)
Instrumental
- Magnetic rack suitable for 0.2 ml PCR tubes
- Microfuge
- Vortex mixer
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
Spin down the Adapter Mix (AMX) and Quick T4 Ligase, and place on ice.
Thaw the Ligation Buffer (LNB) at room temperature, spin down and mix by pipetting. Due to viscosity, vortexing this buffer is ineffective. Place on ice immediately after thawing and mixing.
Thaw the Elution Buffer (EB) at room temperature and mix by vortexing. Then spin down and place on ice.
To retain DNA fragments of all sizes, thaw one tube of Short Fragment Buffer (SFB) at room temperature, mix by vortexing, spin down and place on ice.
In a 1.5 ml Eppendorf DNA LoBind tube, mix in the following order:
| Reagent | Volume |
|---|---|
| End-prepped DNA sample | 60 µl |
| Adapter Mix (AMX) | 5 µl |
| Ligation Buffer (LNB) | 25 µl |
| NEBNext Quick T4 DNA Ligase | 10 µl |
| Total | 100 µl |
Mix gently by flicking the tube and spin down.
Incubate the reaction for 10 minutes at room temperature.
Resuspend the AMPure XP beads by vortexing.
Add 40 µl of resuspended AMPure XP beads to the reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 10 minutes at room temperature.
Spin down the sample and pellet on a magnet. Keep the tube on the magnet, and pipette off the supernatant when clear and colourless.
Wash the beads by adding 200 µl of Short Fragment Buffer (SFB). Flick the beads to resuspend, spin down, then return the tube to the magnetic rack and allow the beads to pellet. Remove the supernatant using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual supernatant. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 15 µl Elution Buffer (EB). Spin down and incubate for 10 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear.
Remove and retain 15 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
The prepared library is used for loading onto the flow cell. Store the library on ice until ready to load.
We recommend loading 5-50 fmol of this final prepared library onto R9.4.1 flow cells or 25-75 fmol onto R10.3 flow cells.
As custom PCR UMI amplicons have short fragment lengths, it is likely sequencing speed drop-off will be observed over the course of the sequencing run. Top up fuel as and when the translocation speed decreases below 300 bases/second. Please refer to the Refuelling your flow cell for further guidance.
Loading more than 50 fmol of DNA can have a detrimental effect on throughput. Dilute the library in Elution Buffer if required.
Library storage recommendations
We recommend storing libraries in Eppendorf DNA LoBind tubes at 4°C for short term storage or repeated use, for example, reloading flow cells between washes. For single use and long-term storage of more than 3 months, we recommend storing libraries at -80°C in Eppendorf DNA LoBind tubes. For further information, please refer to the DNA library stability Know-How document.
If quantities allow, the libraries may be diluted in Elution Buffer (EB) for splitting across multiple flow cells.
Additional buffer for doing this can be found in the Sequencing Auxiliary Vials expansion (EXP-AUX001), available to purchase separately. This expansion also contains additional vials of Sequencing Buffer (SQB) and Loading Beads (LB), required for loading the libraries onto flow cells.
10. Priming and loading the SpotON flow cell
Material
- Flow Cell Priming Kit (EXP-FLP002)
- Loading Beads (LB)
- Sequencing Buffer (SQB)
Consumibles
- 1.5 ml Eppendorf DNA LoBind tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
Instrumental
- MinION Mk1B or Mk1C
- SpotON Flow Cell
- MinION/GridION Flow Cell Light Shield
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
Priming and loading a flow cell
We recommend all new users watch the 'Priming and loading your flow cell' video before your first run.
Thaw the Sequencing Buffer (SQB), Loading Beads (LB), Flush Tether (FLT) and one tube of Flush Buffer (FB) at room temperature before mixing the reagents by vortexing, and spin down at room temperature.
To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Open the MinION device lid and slide the flow cell under the clip.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check instructions in the MinKNOW protocol for more information.
Slide the flow cell priming port cover clockwise to open the priming port.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 µl, to draw back 20-30 µl, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
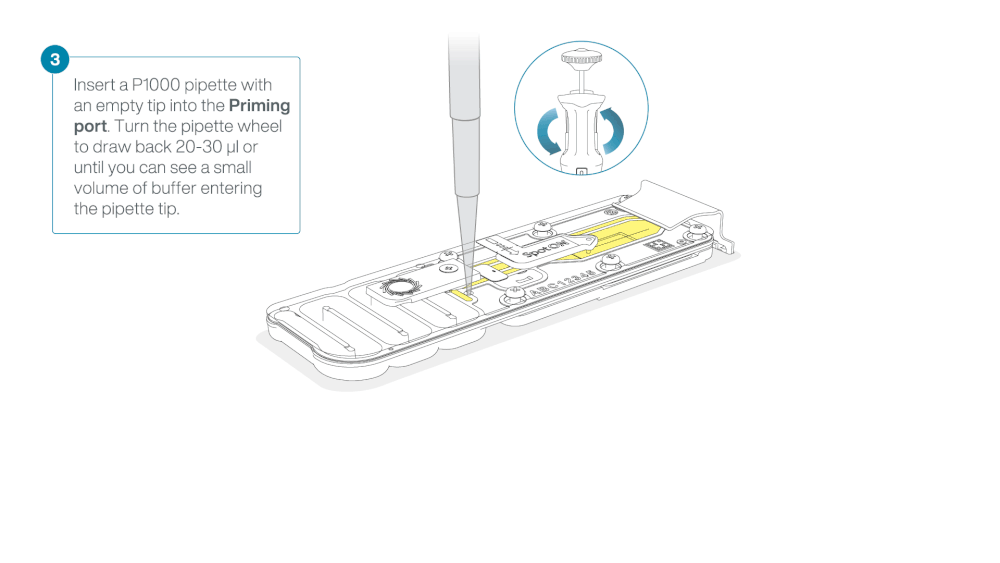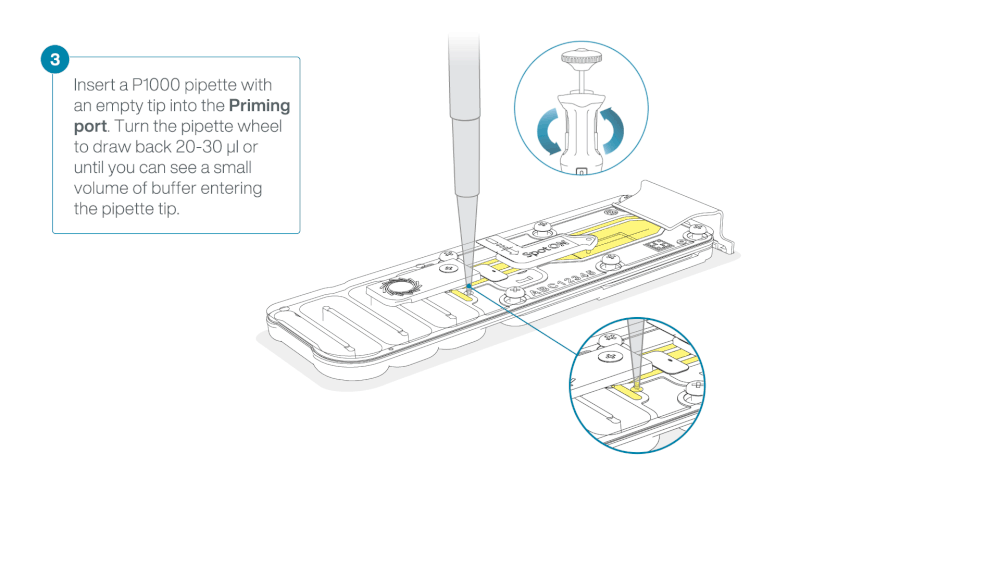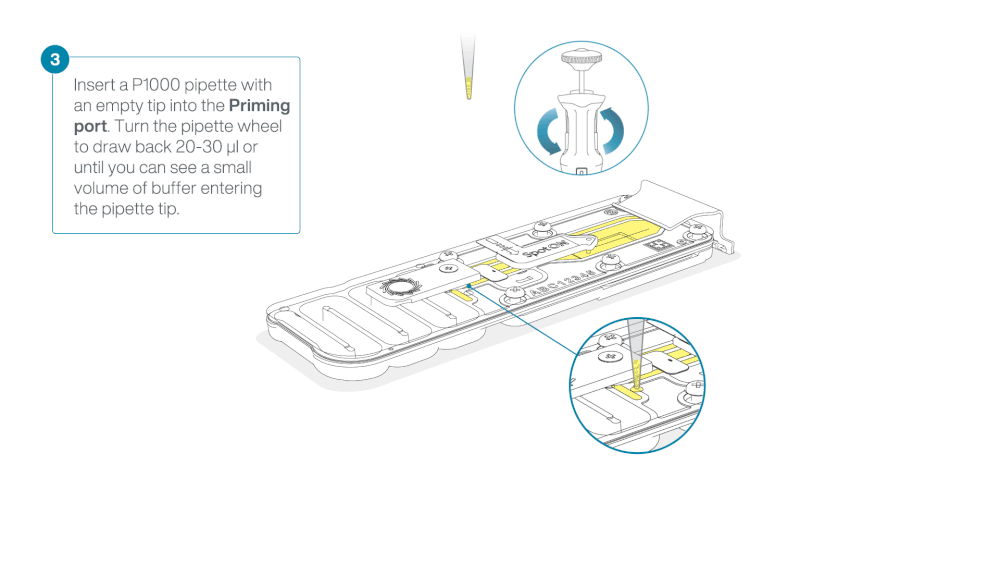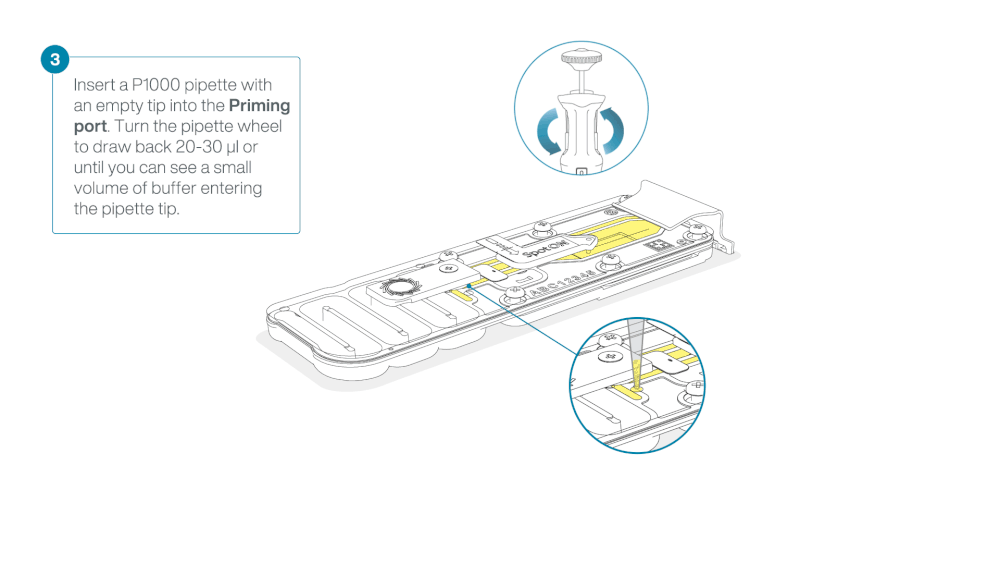
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the Loading Beads (LB) by pipetting.
The Loading Beads (LB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SQB) | 37.5 µl |
| Loading Beads (LB), mixed immediately before use | 25.5 µl |
| DNA library | 12 µl |
| Total | 75 µl |
Note: Load the library onto the flow cell immediately after adding the Sequencing Buffer (SQB) and Loading Beads (LB) because the fuel in the buffer will start to be consumed by the adapter.
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port and close the priming port.

Install the light shield on your flow cell as soon as library has been loaded for optimal sequencing output.
We recommend leaving the light shield on the flow cell when library is loaded, including during any washing and reloading steps. The shield can be removed when the library has been removed from the flow cell.
Place the light shield onto the flow cell, as follows:
Carefully place the leading edge of the light shield against the clip. Note: Do not force the light shield underneath the clip.
Gently lower the light shield onto the flow cell. The light shield should sit around the SpotON cover, covering the entire top section of the flow cell.

The MinION Flow Cell Light Shield is not secured to the flow cell and careful handling is required after installation.
Close the device lid and set up a sequencing run on MinKNOW.
11. Data acquisition and basecalling
Overview of nanopore data analysis
For a full overview of nanopore data analysis, which includes options for basecalling and post-basecalling analysis, please refer to the Data Analysis document.
How to start sequencing
The sequencing device control, data acquisition and real-time basecalling are carried out by the MinKNOW software. Please ensure MinKNOW is installed on your computer or device. There are multiple options for how to carry out sequencing:
1. Data acquisition and basecalling in real-time using MinKNOW on a computer
Follow the instructions in the MinKNOW protocol beginning from the "Starting a sequencing run" section until the end of the "Completing a MinKNOW run" section.
2. Data acquisition and basecalling in real-time using the MinION Mk1B/Mk1D device
Follow the instructions in the MinION Mk1B user manual or the MinION Mk1D user manual.
3. Data acquisition and basecalling in real-time using the MinION Mk1C device
Follow the instructions in the MinION Mk1C user manual.
4. Data acquisition and basecalling in real-time using the GridION device
Follow the instructions in the GridION user manual.
5. Data acquisition and basecalling in real-time using the PromethION device
Follow the instructions in the PromethION user manual or the PromethION 2 Solo user manual.
6. Data acquisition using MinKNOW on a computer and basecalling at a later time using MinKNOW
Follow the instructions in the MinKNOW protocol beginning from the "Starting a sequencing run" section until the end of the "Completing a MinKNOW run" section. When setting your experiment parameters, set the Basecalling tab to OFF. After the sequencing experiment has completed, follow the instructions in the Post-run analysis section of the MinKNOW protocol.
12. Downstream analysis
Recommended analysis pipeline
We currently have a research analysis tool available in the Oxford Nanopore GitHub repository. The pipeline-umi-amplicon workflow is a proof of concept pipeline that creates consensus UMI reads from the raw FASTQ files. This tool is aimed at advanced users, and contains instructions for how to install and run the software.
Overview of analysis
Figure 1. Bioinformatic analysis pipeline overview.
Input
| Input | Type | Description |
|---|---|---|
| Basecalled 1D reads | FASTQ | Basecalled 1D reads |
| Amplicon coordinates | BED | A BED file containing the regions on the reference genome that were targeted |
| Reference genome | FASTA | Reference genome in FASTA format |
Output
| Output | Type | Description |
|---|---|---|
| Aligned UMI reads | BAM | Alignments of UMI reads |
| Variant calls | VCF | Variant calls using varscan2 for UMI reads |
Example data
We have three different example data sets that demonstrate the implementation of the custom PCR UMI protocol from experimental design through to bioinformatic analysis.
Please refer to the Apps Update post Custom PCR UMI example data sets to view the in-depth data.
13. Flow cell reuse and returns
Material
- Kit Flow Cell Wash (EXP-WSH004)
After your sequencing experiment is complete, if you would like to reuse the flow cell, please follow the Flow Cell Wash Kit protocol and store the washed flow cell at +2°C to +8°C.
The Flow Cell Wash Kit protocol is available on the Nanopore Community.
We recommend you to wash the flow cell as soon as possible after you stop the run. However, if this is not possible, leave the flow cell on the device and wash it the next day.
Alternatively, follow the returns procedure to send the flow cell back to Oxford Nanopore.
Instructions for returning flow cells can be found here.
If you encounter issues or have questions about your sequencing experiment, please refer to the Troubleshooting Guide that can be found in the online version of this protocol.
14. Issues during DNA extraction and library preparation
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Low sample quality
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low DNA purity (Nanodrop reading for DNA OD 260/280 is <1.8 and OD 260/230 is <2.0–2.2) | The DNA extraction method does not provide the required purity | The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. Consider performing an additional SPRI clean-up step. |
Low DNA recovery after AMPure bead clean-up
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low recovery | DNA loss due to a lower than intended AMPure beads-to-sample ratio | 1. AMPure beads settle quickly, so ensure they are well resuspended before adding them to the sample. 2. When the AMPure beads-to-sample ratio is lower than 0.4:1, DNA fragments of any size will be lost during the clean-up. |
| Low recovery | DNA fragments are shorter than expected | The lower the AMPure beads-to-sample ratio, the more stringent the selection against short fragments. Please always determine the input DNA length on an agarose gel (or other gel electrophoresis methods) and then calculate the appropriate amount of AMPure beads to use. 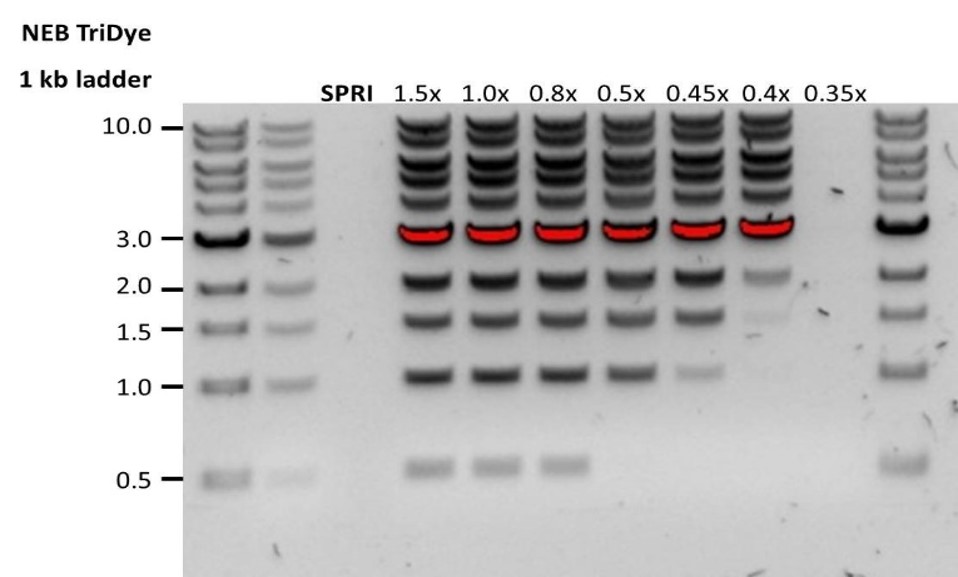 |
| Low recovery after end-prep | The wash step used ethanol <70% | DNA will be eluted from the beads when using ethanol <70%. Make sure to use the correct percentage. |
15. Issues during the sequencing run
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Fewer pores at the start of sequencing than after Flow Cell Check
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | An air bubble was introduced into the nanopore array | After the Flow Cell Check it is essential to remove any air bubbles near the priming port before priming the flow cell. If not removed, the air bubble can travel to the nanopore array and irreversibly damage the nanopores that have been exposed to air. The best practice to prevent this from happening is demonstrated in this video. |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | The flow cell is not correctly inserted into the device | Stop the sequencing run, remove the flow cell from the sequencing device and insert it again, checking that the flow cell is firmly seated in the device and that it has reached the target temperature. If applicable, try a different position on the device (GridION/PromethION). |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | Contaminations in the library damaged or blocked the pores | The pore count during the Flow Cell Check is performed using the QC DNA molecules present in the flow cell storage buffer. At the start of sequencing, the library itself is used to estimate the number of active pores. Because of this, variability of about 10% in the number of pores is expected. A significantly lower pore count reported at the start of sequencing can be due to contaminants in the library that have damaged the membranes or blocked the pores. Alternative DNA/RNA extraction or purification methods may be needed to improve the purity of the input material. The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
MinKNOW script failed
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Script failed" | Restart the computer and then restart MinKNOW. If the issue persists, please collect the MinKNOW log files and contact Technical Support. If you do not have another sequencing device available, we recommend storing the flow cell and the loaded library at 4°C and contact Technical Support for further storage guidance. |
Pore occupancy below 40%
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Pore occupancy <40% | Not enough library was loaded on the flow cell | Ensure you load the recommended amount of good quality library in the relevant library prep protocol onto your flow cell. Please quantify the library before loading and calculate mols using tools like the Promega Biomath Calculator, choosing "dsDNA: µg to pmol" |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and sequencing adapters did not ligate to the DNA | Make sure to use the NEBNext Quick Ligation Module (E6056) and Oxford Nanopore Technologies Ligation Buffer (LNB, provided in the sequencing kit) at the sequencing adapter ligation step, and use the correct amount of each reagent. A Lambda control library can be prepared to test the integrity of the third-party reagents. |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and ethanol was used instead of LFB or SFB at the wash step after sequencing adapter ligation | Ethanol can denature the motor protein on the sequencing adapters. Make sure the LFB or SFB buffer was used after ligation of sequencing adapters. |
| Pore occupancy close to 0 | No tether on the flow cell | Tethers are adding during flow cell priming (FLT/FCT tube). Make sure FLT/FCT was added to FB/FCF before priming. |
Shorter than expected read length
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Shorter than expected read length | Unwanted fragmentation of DNA sample | Read length reflects input DNA fragment length. Input DNA can be fragmented during extraction and library prep. 1. Please review the Extraction Methods in the Nanopore Community for best practice for extraction. 2. Visualise the input DNA fragment length distribution on an agarose gel before proceeding to the library prep. 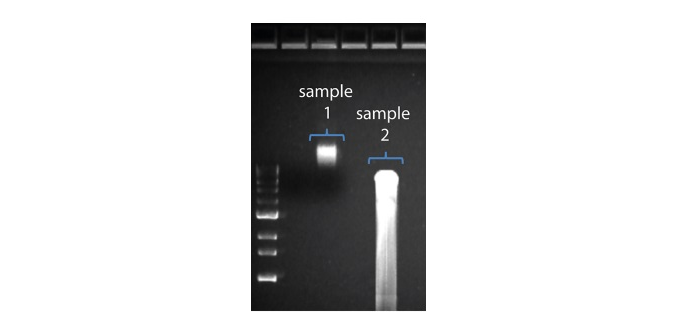 In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented. In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented.3. During library prep, avoid pipetting and vortexing when mixing reagents. Flicking or inverting the tube is sufficient. |
Large proportion of unavailable pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
Large proportion of unavailable pores (shown as blue in the channels panel and pore activity plot) 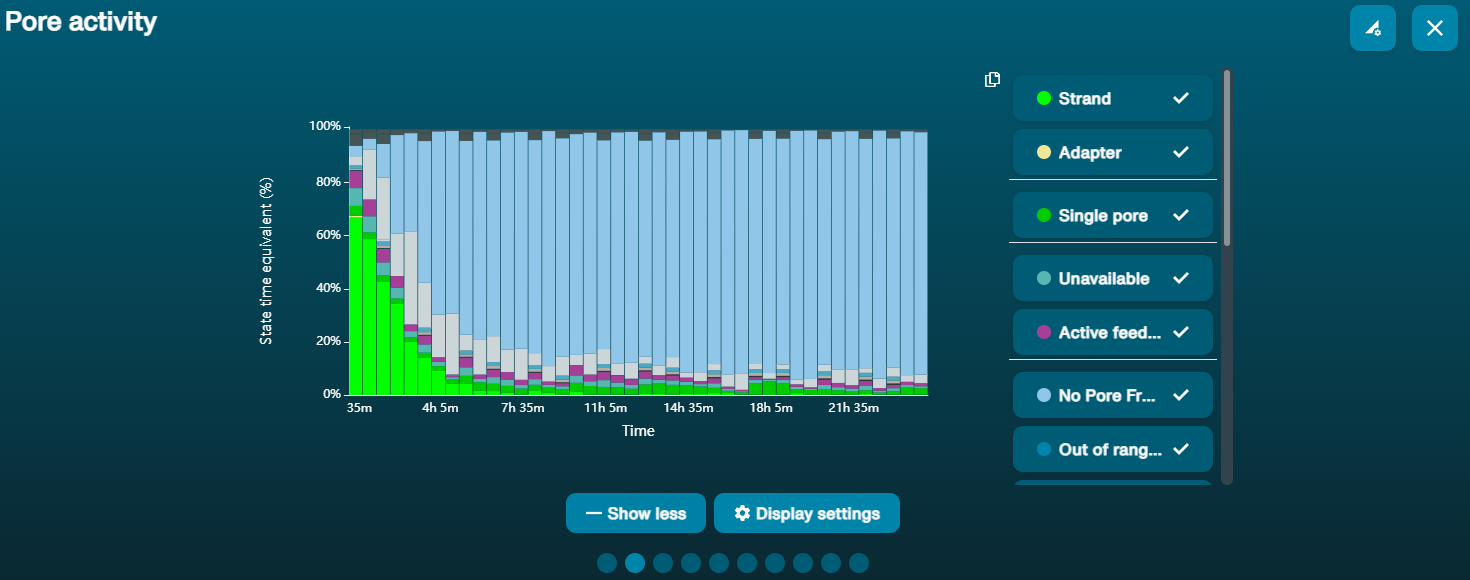 The pore activity plot above shows an increasing proportion of "unavailable" pores over time. The pore activity plot above shows an increasing proportion of "unavailable" pores over time. | Contaminants are present in the sample | Some contaminants can be cleared from the pores by the unblocking function built into MinKNOW. If this is successful, the pore status will change to "sequencing pore". If the portion of unavailable pores stays large or increases: 1. A nuclease flush using the Flow Cell Wash Kit (EXP-WSH004) can be performed, or 2. Run several cycles of PCR to try and dilute any contaminants that may be causing problems. |
Large proportion of inactive pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Large proportion of inactive/unavailable pores (shown as light blue in the channels panel and pore activity plot. Pores or membranes are irreversibly damaged) | Air bubbles have been introduced into the flow cell | Air bubbles introduced through flow cell priming and library loading can irreversibly damage the pores. Watch the Priming and loading your flow cell video for best practice |
| Large proportion of inactive/unavailable pores | Certain compounds co-purified with DNA | Known compounds, include polysaccharides, typically associate with plant genomic DNA. 1. Please refer to the Plant leaf DNA extraction method. 2. Clean-up using the QIAGEN PowerClean Pro kit. 3. Perform a whole genome amplification with the original gDNA sample using the QIAGEN REPLI-g kit. |
| Large proportion of inactive/unavailable pores | Contaminants are present in the sample | The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
Reduction in sequencing speed and q-score later into the run
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Reduction in sequencing speed and q-score later into the run | For Kit 9 chemistry (e.g. SQK-LSK109), fast fuel consumption is typically seen when the flow cell is overloaded with library (please see the appropriate protocol for your DNA library to see the recommendation). | Add more fuel to the flow cell by following the instructions in the MinKNOW protocol. In future experiments, load lower amounts of library to the flow cell. |
Temperature fluctuation
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Temperature fluctuation | The flow cell has lost contact with the device | Check that there is a heat pad covering the metal plate on the back of the flow cell. Re-insert the flow cell and press it down to make sure the connector pins are firmly in contact with the device. If the problem persists, please contact Technical Services. |
Failed to reach target temperature
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Failed to reach target temperature" | The instrument was placed in a location that is colder than normal room temperature, or a location with poor ventilation (which leads to the flow cells overheating) | MinKNOW has a default timeframe for the flow cell to reach the target temperature. Once the timeframe is exceeded, an error message will appear and the sequencing experiment will continue. However, sequencing at an incorrect temperature may lead to a decrease in throughput and lower q-scores. Please adjust the location of the sequencing device to ensure that it is placed at room temperature with good ventilation, then re-start the process in MinKNOW. Please refer to this link for more information on MinION temperature control. |
Guppy – no input .fast5 was found or basecalled
| Observation | Possible cause | Comments and actions |
|---|---|---|
| No input .fast5 was found or basecalled | input_path did not point to the .fast5 file location | The --input_path has to be followed by the full file path to the .fast5 files to be basecalled, and the location has to be accessible either locally or remotely through SSH. |
| No input .fast5 was found or basecalled | The .fast5 files were in a subfolder at the input_path location | To allow Guppy to look into subfolders, add the --recursive flag to the command |
Guppy – no Pass or Fail folders were generated after basecalling
| Observation | Possible cause | Comments and actions |
|---|---|---|
| No Pass or Fail folders were generated after basecalling | The --qscore_filtering flag was not included in the command | The --qscore_filtering flag enables filtering of reads into Pass and Fail folders inside the output folder, based on their strand q-score. When performing live basecalling in MinKNOW, a q-score of 7 (corresponding to a basecall accuracy of ~80%) is used to separate reads into Pass and Fail folders. |
Guppy – unusually slow processing on a GPU computer
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Unusually slow processing on a GPU computer | The --device flag wasn't included in the command | The --device flag specifies a GPU device to use for accelerate basecalling. If not included in the command, GPU will not be used. GPUs are counted from zero. An example is --device cuda:0 cuda:1, when 2 GPUs are specified to use by the Guppy command. |







