Restriction enzyme Pore-C protocol for plant samples
Extraction Method
Restriction enzyme Pore-C protocol for plant samples
FOR RESEARCH USE ONLY
Contents
Introduction
Materials
Method
- 1. Day 1: 1 hour hands-on time
- 2. Cryogrinding: 20 minutes hands-on time
- 3. Nuclei isolation: 1.5 hours hands-on time, 1.5 hours procedure
- 4. Chromatin denaturation: 10 minutes hands-on time, 40 minutes procedure
- 5. Day 2: 5–10 minutes hands-on time, 6-hour procedure time + overnight
- 6. Proximity ligation A with heat denaturation: 5 minutes hands-on time, 6-hour procedure
- 7. Proximity ligation B with heat denaturation: 5 minutes hands-on time, 6-hour procedure
- 8. Protein degradation and de-crosslinking: 5 minutes hands-on time, 5 minutes procedure
- 9. Day 3: DNA extraction: 40 minutes hands-on time, 110 minutes procedure
Library preparation
Sequencing experiment
Results
Change log
Introduction
The plant restriction enzyme Pore-C (RE-Pore-C) protocol is intended for the manipulation of plant nuclei suspensions to capture three‑dimensional interactions of DNA within chromatin. We strongly recommend reading the Restriction Enzyme Pore-C info sheet prior to starting thisprotocol to understand how to choose the appropriate restriction enzyme, the sequencing kit for library preparation, and information regarding sample input.
This protocol has been developed based on research by Oxford Nanopore Technologies and published literature:
- Belaghzal, H., Dekker, J. and Gibcus, J. H. (2017) Hi-C 2.0: an optimized Hi-C procedure for high-resolution genome-wide mapping of chromosome conformation, Methods, 123, pp. 56–65. doi: 10.1016/j.ymeth.2017.04.004.HI-C.
- Belton, J.-M. et al. (2012) Hi-C: a comprehensive technique to capture the conformation of genomes, Methods, 58(3), pp. 1–16. doi: 10.1016/j.ymeth.2012.05.001.Hi-C.
- Comet, I. et al. (2011) A chromatin insulator driving three-dimensional Polycomb response element (PRE) contacts and Polycomb association with the chromatin fiber, Proceedings of the National Academy of Sciences, 108(6), pp. 2294–2299. doi: 10.1073/pnas.1002059108.
- Folta, K. M. and Kaufman, L. S. (2006) ‘Isolation of Arabidopsis nuclei and measurement of gene transcription rates using nuclear run-on assays’, Nature Protocols, 1(6), pp. 3094–3100.
- Gavrilov, A. A., Golov, A. K. and Razin, S. V. (2013) Actual Ligation Frequencies in the Chromosome Conformation Capture Procedure, PLoS ONE, 8(3), pp. 1–6. doi: 10.1371/journal.pone.0060403.
- Lieberman-Aiden, E. et al. (2009) Comprehensive mapping of long range interactions reveals folding principles of the human genome, Science, 326, pp. 289–293.
- Liu, C. (2017) ‘Plant Gene Regulatory Networks’, in Plant Gene Regulatory Networks: Methods and Protocols, Methods in Molecular Biology, pp. 155–166. doi: 10.1007/978-1-4939-7125-1.
- Nagano, T. et al. (2015) Comparison of Hi-C results using in-solution versus in-nucleus ligation, Genome Biology, 16(1), pp. 1–13. doi: 10.1186/s13059-015-0753-7.
- Ulahannan, N. et al. (2019) Nanopore sequencing of DNA concatemers reveals higher-order features of chromatin structure, bioRxiv, p. 833590; doi: 10.1101/833590.
- Workman, R. et al. (2018) ‘High molecular weight DNA extraction from recalcitrant plant species for third generation sequencing’, Protocol Exchange, version 1, pp. 1–15. doi: 10.1038/protex.2018.059.
- Zhang, M. et al. (2012) ‘Preparation of megabase-sized DNA from a variety of organisms using the nuclei method for advanced genomics research’, Nature Protocols. Nature Publishing Group, 7(3), pp. 467–478. doi: 10.1038/nprot.2011.455.
Materials
- 2 g of plant material
- An appropriate restriction enzyme and associated buffer; for example NlaIII restriction enzyme (or equivalent) at 10 U/μl (NEB, R0125L) and NlaIII reaction buffer
- 0.5 M EDTA, pH 8
- Formaldehyde at 36.5% v/v (Sigma, F8775)
- Pure ethanol
- Glycine (Sigma, 50046-250G)
- 5 M NaCl (Sigma, 71386)
- 10X phosphate-buffered saline (PBS)
- Phenol:chloroform:isoamyl alcohol in a 25:24:1 ratio, saturated with 10 mM Tris.HCl pH 8.0, 1 mM EDTA (Sigma P3803-400ML)
- Polyvinylpyrrolidone (PVP) PVP-40 (Sigma PVP40)
- Protease Inhibitor Cocktail (Sigma, P8340)
- Proteinase K at 20 μg/μl (NEB, P8107S)
- Recombinant albumin at 20 μg/μl (NEB, B9200S)
- SDS at 10% v/v (Fisher, 10265153)
- 3 M sodium acetate, pH 5.5
- Spermidine trihydrochloride (Sigma, 85578)
- Spermine tetrahydrochloride (Sigma, 85605)
- Sucrose (Sigma, S0389)
- T4 DNA Ligase 400,000 U/ml (NEB M0202S/L)
- 1 M Tris-HCl pH 8.0 (Fisher, 10336763)
- ECOSURF™ EH-9 (Sigma, STS0006) or Triton X-100 (Sigma, X100)
- Trizma base (Sigma, 93362)
- Tween-20 (Sigma, P9416)
- β-mercaptoethanol at 14.3 M (Sigma, 63689)
- Qubit dsDNA HS Assay Kit (ThermoFisher Q32851)
- Liquid nitrogen
- 1 ml disposable syringe
- 1.5 ml Eppendorf DNA LoBind tubes
- 2 ml Eppendorf DNA LoBind tubes
- 5 ml centrifuge tubes
- 50 ml centrifuge tubes
- Centrifuge and rotor for 15 ml Falcon tubes
- Ice bucket with ice
- 4°C and –80°C freezer storage
- Microfuge
- Microfuge rotor for 5 ml tubes
- Microfuge rotor for 2 ml tubes
- Microfuge rotor for 50 ml tubes
- Thermomixer with temperature control and rotation
- 10, 20, 200 and 1000 μl filter pipette tips and 100, 200 and 1000 μl wide-bore pipette tips
- 2, 10, 20, 200 and 1000 μl pipettes
- 40 μm cell strainer (Fisher, 11587522)
- 100 ml glass measuring cylinder
- 500 ml borosilicate filtering flask
- 500 ml borosilicate round bottom flask
- Büchner funnel with sintered glass filter
- Class I hood with active charcoal filter
- Gyratory rocker
- Rotary evaporator system (rotovap) with pressure-controlled vacuum
- Qubit fluorometer (or equivalent for QC check)
- Agilent Bioanalyzer and 12,000 bp reagents (or equivalent)
Method
Day 1: 1 hour hands-on time
Note: Formaldehyde crosslinking of chromatin is achieved with vacuum infiltration. In this protocol, a rotary evaporator system (rotovap) is used to limit boiling. We recommend using a rotovap for the vacuum infiltration procedure, however users may also apply vacuum to a static inside of a vacuum chamber or desiccator.
1. Prepare 10 ml of filtered 2.5 M glycine.
2. Prepare 400 ml of fresh vacuum infiltration buffer and store at 4°C prior to use.
| Component | Stock conc. | Final conc. | For 400 ml |
|---|---|---|---|
| PBS pH 7.4 | 10X | 1X | 40 ml |
| Sucrose | - | 100 mM | 13.69 g |
| ddH2O | - | - | Up to 400 ml |
| Total | - | - | 400 ml |
3. Collect 2 g of plant material. We recommend selecting younger leaves.
Note: If the plant material is >1 cm^2, dissect the sample into smaller pieces before proceeding to the next step. Frozen plant material may also be thawed for use, however we recommend fresh material
4. In a 500 ml round-bottom flask, suspend the dissected plant material in 100 ml of vacuum infiltration buffer.
5. To reduce the surface tension of the buffer and prevent the plant material from clumping together, add 100 µl of 10% v/v ECOSURF™ EH-9 to the suspension for a final concentration of 0.01% v/v.
6. Continue all subsequent steps inside a class I hood with double gloves. Add 2910 µl of 36.5% v/v formaldehyde to the suspension for a final concentration of 1% v/v formaldehyde in ~103 ml.
7. Connect the round-bottom flask to the rotovap and apply a 16 kPa (160 mBar) vacuum with 100 rpm rotation for 15 minutes at room temperature.
8. Release the vacuum to atmospheric pressure, then reapply a 16 kPa (160 mBar) vacuum with 100 rpm rotation for a further 15 minutes at room temperature.
9. Release the vacuum to atmospheric pressure, then decant the suspension into a Büchner funnel with a sintered glass filter. Vacuum-filter the suspension over a 500 ml filtering flask.
10. Remove the Büchner funnel from the filtering flask and discard the filtrate. Resuspend the plant material in 100 ml of fresh vacuum infiltration buffer. Decant the suspension into the 500 ml round-bottom flask.
11. Add 5270 μl of 2.5 M glycine to the suspension for a final concentration of 1% w/v glycine (125 mM) in ~105 ml and mix by swirling.
12. Connect the round-bottom flask to the rotovap and apply a 16 kPa (160 mBar) vacuum with 100 rpm rotation for 10 minutes at room temperature.
13. Release the vacuum to atmospheric pressure, then decant the suspension into a Büchner funnel with a sintered glass filter. Rinse out the round-bottom flask with distilled water and also decant into the Büchner funnel. Vacuum-filter the suspension over a 500 ml filtering flask.
14. Remove the Büchner funnel from the filtering flask and discard the filtrate. Wash the plant material retained in the Büchner funnel twice with 50 ml of fresh vacuum infiltration buffer for 5 minutes each prior to filtering.
15. Remove the Büchner funnel from the filtering flask and discard the filtrate. Wash the plant material retained in the Büchner funnel with 50 ml of distilled water and filter again.
16. Remove the Büchner funnel from the filtering flask and discard the filtrate. Using a spatula, transfer the plant material into a fresh 50 ml centrifuge tube.
17. The crosslinked plant material may now be removed from the class I hood. We recommend continuing directly to cryogrinding. However, the crosslinked plant material may be snap‑frozen in liquid nitrogen then stored at –80°C for later use.
Cryogrinding: 20 minutes hands-on time
1. Pre-cool the mortar and pestle at –80°C for at least 30 minutes.
2. Place the chilled mortar and pestle on ice. Add a small volume of liquid nitrogen into the mortar, then add the cross-linked plant material briefly and allow to freeze until the liquid nitrogen has evaporated.
3. Carefully grind the cross-linked plant material into a fine powder, working quickly to minimise thawing. If the material starts to thaw, freeze further by adding a small volume of liquid nitrogen to the mortar.
4. Use a spatula to collect the cryo-ground powder into a chilled 50 ml centrifuge tube on ice. We recommend continuing directly to nuclei isolation.
5. Optional Step If needed, the cryo-ground cross-linked plant material may be snap‑frozen in liquid nitrogen then stored at –80°C for later use.
Nuclei isolation: 1.5 hours hands-on time, 1.5 hours procedure
Note: Do not proceed any further unless it is possible to complete the remainder of the protocol consecutively without delay. At no point is it advisable to hold any step longer than stated in this protocol. Increasing the timing of steps will be detrimental to Pore-C data quality and sequencing performance.
1. Pre-cool a 50 ml centrifuge to 4°C.
2. Prepare the following buffers:
1. 10 ml of 1X PBS and store at 4°C
2. 10X homogenisation buffer:
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| Trizma-base | - | 100 mM | 0.61 g |
| KCl | - | 800 mM | 3.00 g |
| EDTA | 0.5 M | 100 mM | 10 ml |
| Spermidine trihydrochloride | - | 10 mM | 0.13 g |
| Spermine tetrahydrochloride | - | 10 mM | 0.17 g |
| ddH2O | - | - | Up to 50 ml |
| Total | - | - | 50 ml |
Note: Adjust the pH to 9.0 - 9.4 with NaOH. Store at 4°C prior to use.
3. Nuclei isolation buffer:
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| 10X homogenisation buffer | 10X | 1X | 10 ml |
| Sucrose | - | 500 mM | 17.12 g |
| PVP-40 | - | 1% w/v | 1.00 g |
| ECOSURF™ EH-9 | 100% | 0.5% v/v | 500 µl |
| ddH2O | - | - | Up to 100 ml |
| β-mercaptoethanol | 100% (14.3 M) | 0.25% v/v final (35.75 mM) | 250 µl |
| Total | - | - | 100 ml |
Note: Do not add the β-mercaptoethanol until ready to use. Store at 4°C prior to use. Alternatively to ECOSURF™ EH-9, Triton X-100 may be used.
4. Washing buffer:
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| 10X homogenisation buffer | 10X | 1X | 1 ml |
| Sucrose | - | 500 mM | 1.71 g |
| ddH2O | - | - | Up to 10 ml |
| Total | - | - | 10 ml |
Note: Store at 4°C prior to use.
5. Resuspend 1.5–2 g of cryo-ground plant material in 20 ml of chilled nuclei isolation buffer in a 50 ml centrifuge tube.
Note: Ensure the buffer has been supplemented with β-mercaptoethanol to 0.25% v/v final.
6. Ensure the centrifuge tube lid is fastened and place the sample horizontally on ice and mix on a gyratory rocker at 50 rpm for 15 minutes.
7. Pass the suspension through a 40 μm cell strainer into a fresh chilled 50 ml tube on ice.
8. Once the sample has passed through the filter, rinse the original sample tube with a further 5 ml of chilled nuclei isolation buffer and filter through the same 40 μm cell strainer.
9. Centrifuge for 20 minutes at 4°C using a centrifugation force appropriate for the genome size (see Figure 1):
| Genome size (Mb) | Centrifugation force (g) |
|---|---|
| 25 | 4800 |
| 50 | 4370 |
| 100 | 3970 |
| 250 | 3500 |
| 500 | 3190 |
| 1,000 | 2900 |
| 2,500 | 2560 |
| 5,000 | 2330 |
| 10,000 | 2120 |
| 25,000 | 1870 |
| 50,000 | 1700 |
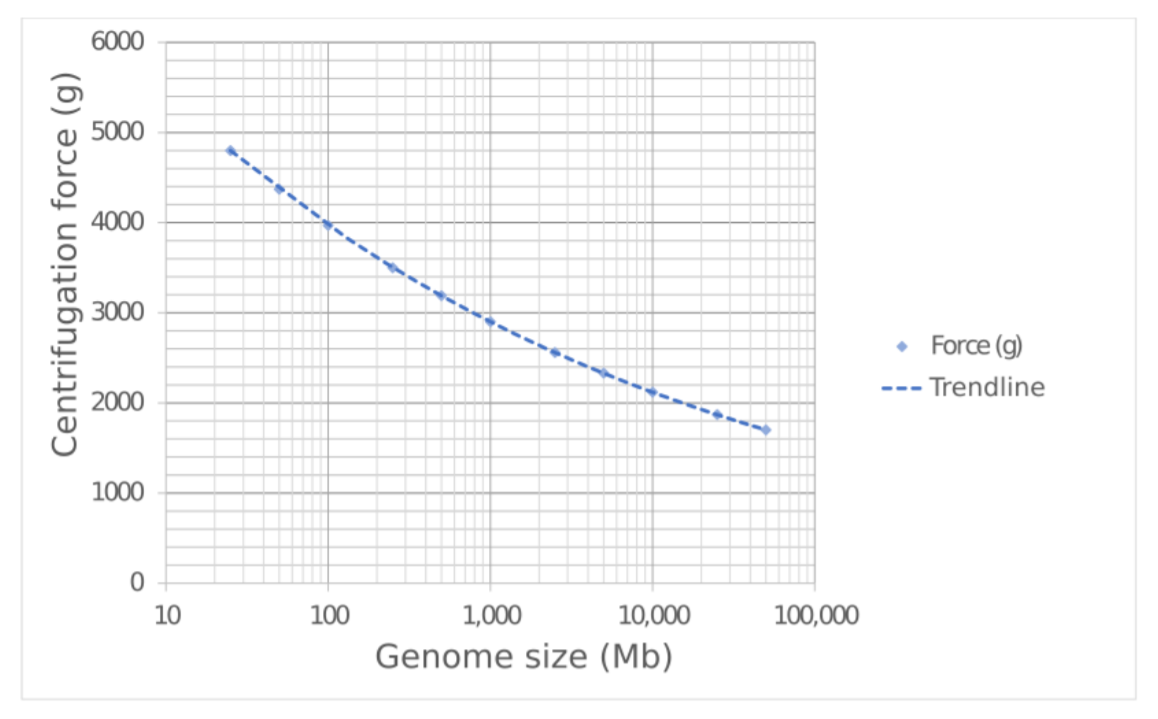 Figure 1. Recommended centrifugation force for nuclei isolation according to genome size.
Figure 1. Recommended centrifugation force for nuclei isolation according to genome size.
10. Carefully decant and discard the supernatant. Using a wide-bore pipette tip, gently resuspend the pellet in 1 ml of chilled nuclei isolation buffer.
11. Add a further 19 ml chilled nuclei isolation buffer to the suspension and mix by swirling.
12. Centrifuge for a further 10 minutes at 4°C using a centrifugation force appropriate for the genome size (see Figure 1).
13. Repeat the three steps above (steps 10–12 from this section of the document).
14. Pre-cool a 2 ml centrifuge to 4°C.
15. Carefully decant and discard the supernatant. Using a wide-bore pipette tip, gently resuspend the pellet in 1 ml of cold washing buffer. Transfer the sample to a fresh 2 ml centrifuge tube on ice. Rinse the original tube with a further 1 ml of cold washing buffer and transfer to the same 2 ml centrifuge tube.
16. Centrifuge for 5 minutes at 4°C using a centrifugation force appropriate for the genome size.
17. Aspirate and discard as much of the supernatant as possible without disturbing the pellet. Using a wide-bore pipette tip, gently resuspend the pellet in 2 ml of chilled 1X PBS.
18. Centrifuge for 5 minutes at 4°C at 3000 g.
19. Aspirate and discard the supernatant.
Chromatin denaturation: 10 minutes hands-on time, 40 minutes procedure
Note: Pre-heat thermomixer to 62°C.
1. Resuspend the pellet of crosslinked plant nuclei in the 100 μl of 0.5% SDS and homogenise by gentle pipetting with a wide-bore pipette tip.
2. Incubate at 62°C for 6 minutes without agitation, then cool to room temperature.
3. Pre-heat the thermomixer to 37°C.
4. Add 250 μl of nuclease-free water and 50 μl of 10% v/v ECOSURF™ EH-9 directly to the plant nuclei suspension for a final concentration of 1.25% v/v ECOSURF™ EH-9 in 400 μl. Mix by gentle pipetting with a wide-bore pipette tip.
Note: Alternatively to ECOSURF™ EH-9, Triton X-100 may be used.
5. Incubate at 37°C for 15 minutes without agitation.
6. Following incubation, the chromatin is ready for digestion. Add the following reagents to the plant nuclei suspension to achieve a final concentration of 1 U/μl for the selected restriction enzyme. Example NlaIII digestion:
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| Plant nuclei suspension | - | - | 400 μl |
| NEB CutSmart buffer | 10X | 1X | 50 μl |
| NEB NlaIII | 10 U/μl | 1 U/μl | 50 μl |
| Total | - | - | 500 μl |
7. Mix by gentle inversion and incubate the plant nuclei suspension in a thermomixer at 37°C for 18 hours without agitation before proceeding directly to proximity ligation.
Day 2: 5–10 minutes hands-on time, 6-hour procedure time + overnight
Depending on the restriction enzyme selected for the previous in situ digestion, follow one of the appropriate ligation reactions below then continue with the rest of the protocol for Day 2.
Do not attempt both ligation reactions using a single Pore-C extraction.
The proximity ligation reaction protocol selected will depend on the restriction enzyme used:
- If in situ digestion was carried out using a restriction enzyme that can be heat-denatured at <65°C, then proceed with proximity ligation A with heat denaturation.
- If the in situ digestion was carried out using an enzyme that cannot be heat-denatured at <65°C, then proceed with proximity ligation B with chemical denaturation.
It is not advisable to attempt heat denaturation at temperatures >65°C, as this may result in unintended crosslink reversal prior to proximity ligation.
Proximity ligation A with heat denaturation: 5 minutes hands-on time, 6-hour procedure
Note: Pre-heat the thermomixer to 65°C.
1. Heat-denature the restriction enzyme by incubating the plant nuclei suspension in a thermomixer at 65°C for 20 minutes at 300 rpm rotation.
2. Pre-cool the thermomixer to 16°C. Allow the plant nuclei suspension to cool to room temperature (~21°C) and set up the proximity ligation reaction according to the table below. Add the reagents in the following order directly to the plant nuclei suspension.
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| Digestion reaction | - | - | 500 µl |
| Nuclease-free water | - | - | 345 µl |
| NEB ligase buffer | 10X | 1X | 100 µl |
| Recombinant albumin | 20 µg/µl | 0.1 µg/µl | 5 µl |
| T4 DNA ligase | 400 U/µl | 20 U/µl | 50 µl |
| Total | - | - | 1000 µl |
3. Mix by gentle pipetting with a wide-bore pipette tip, then incubate the plant nuclei suspension in a thermomixer at 16°C for 6 hours without agitation.
Note: Prolonged ligation may increase trans-chromosomal contacts in the Pore-C data.
4. Once the incubation is finished, completing ligation, continue directly to protein degradation and de-crosslinking.
Proximity ligation B with heat denaturation: 5 minutes hands-on time, 6-hour procedure
Note: Pre-heat the thermomixer to 65°C.
1. Chemically denature the restriction enzyme by adding 55 μl 1% SDS directly to the plant nuclei suspension for a final concentration of 0.1% SDS in a final volume of 555 μl. Mix by gently pipetting with a wide-bore pipette tip. Then incubate the plant nuclei suspension in a thermomixer at 65°C for 20 minutes without agitation.
2. Cool the thermomixer to 16°C. Allow the plant nuclei to cool to room temperature (~21°C) and set up the proximity ligation reaction according to the table below. Add the reagents in the following order directly to the plant nuclei suspension and mixing by gentle inversion after every addition.
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| Digestion reaction | - | - | 555 µl |
| Nuclease-free water | - | - | 190 µl |
| ECOSURF™ EH-9 | 10% | 1% | 100 µl |
| NEB ligase buffer | 10X | 1X | 100 µl |
| Recombinant albumin | 20 µg/µl | 0.1 µg/µl | 5 µl |
| T4 DNA ligase | - | ~5% | 50 µl |
| Total | - | - | 1000 µl |
Note: Alternatively to ECOSURF™ EH-9, Triton X-100 may be used.
3. Mix by gentle pipetting with a wide-bore pipette tip and incubate the plant nuclei suspension in a thermomixer at 16°C for 6 hours without agitation.
Note: Prolonged ligation may increase trans-chromosomal contacts in Pore-C data.
4. Once the incubation is finished, completing ligation, continue directly to protein degradation and de-crosslinking.
Protein degradation and de-crosslinking: 5 minutes hands-on time, 5 minutes procedure
Note: Pre-heat the thermomixer to 56°C.
1. To set up the protein degradation reaction, add the following reagents to the previous ligation reaction:
| Component | Stock conc. | Final conc. | Volume |
|---|---|---|---|
| Ligation reaction | - | - | 1000 µl |
| Nuclease-free water | - | - | 300 µl |
| Tween-20 | 20% | 5% | 500 µl |
| SDS | 10% | 0.5% | 100 µl |
| Proteinase K | 20 µg/µl | 1 µg/µl | 100 µl |
| Total | - | - | 2000 µl |
2. Mix by gentle inversion. Incubate the plant nuclei suspension in a thermomixer at 56°C for 18 hours with periodic <1000 rpm rotation (<30 seconds every 15 minutes) to prevent condensation inside the lid.
Note: Incubation at 56°C is an ideal compromise of enzyme activity over a prolonged period. It is not advisable to incubate at higher temperatures as enzyme activity will reduce over time.
Day 3: DNA extraction: 40 minutes hands-on time, 110 minutes procedure
Note: Pre-cool a 5 ml centrifuge to 15°C.
1. Cool the protein degradation reaction on ice, then transfer the entire volume to a 5 ml centrifuge tube. Rinse the original tube with a further 200 μl of nuclease‑free water and transfer this to the 5 ml centrifuge tube.
2. The total sample volume should be approximately 2200 μl. Add an equal volume of chilled phenol:chloroform:isoamyl alcohol (25:24:1) saturated with 10 mM Tris.HCl pH 8.0 1 mM EDTA.
Note: Adjust this volume as needed to match that of the sample.
3. Mix with gentle inversion for 5 minutes to achieve a homogenous emulsion.
4. Centrifuge the sample at 15,000 g, 15°C for 15 minutes.
5. Carefully aspirate 2000 μl of the aqueous phase and transfer to a fresh 5 ml centrifuge tube. Add an equal volume of chilled phenol:chloroform:isoamyl alcohol (25:24:1) saturated with 10 mM Tris.HCl pH 8.0 1 mM EDTA.
Note: Adjust this volume as needed to match that of the sample.
6. Mix with gentle inversion for 5 minutes to achieve a homogenous emulsion.
7. Centrifuge the sample at 15,000 g, 15°C for 15 minutes.
8. Transfer the aqueous phase to a fresh 5 ml centrifuge tube and make a note of the recovered volume (~2000 μl).
Note: Be cautious not to take any of the interphase layer.
9. Transfer half of the recovered aqueous phase to a second 5 ml centrifuge tube to create two equal aliquots.
10. Add 0.02 volumes of 5 M NaCl (0.1 M final) and 0.1 volumes of 3 M sodium acetate pH 5.5 (0.3 M final) relative to the volume of the recovered aqueous phase in Step 8 to both 5 ml centrifuge tubes. Mix by gentle agitation and the solution will likely turn cloudy and then clear.
11. Add 3 volumes of 100% EtOH relative to the volume of the recovered aqueous phase in Step 8 to both 5 ml centrifuge tubes. Mix by gentle inversion and precipitate at –80°C for >1 hour or overnight at –20°C if possible.
12. Pre-cool the centrifuge to 4°C.
13. Centrifuge the samples in the 5 ml centrifuge tubes at 16,000 g, 4°C for 30 minutes.
14. Aspirate and discard the supernatant before washing the pellets in 4 ml 80% EtOH.
15. Centrifuge both samples at 16,000 g, 4°C for 5 minutes.
16. Aspirate and discard the supernatant before washing the pellets in 2 ml 70% EtOH.
17. Centrifuge the samples at 16,000 g, 4°C for 5 minutes.
18. Aspirate and discard the supernatant. Briefly spin down the tubes and aspirate any residual supernatant, then allow the pellets to air-dry for 5 minutes.
Note: After the pellets have air-dried, they may become loose in the tube.
19. Carefully resuspend both pellets individually in 75 μl TE buffer and incubate for 5 minutes at room temperature (~21°C). Mix by gentle agitation every few minutes.
20. Briefly spin down the tubes before transferring and pooling both samples into a 1.5 ml microcentrifuge tube.
21. Quantify the DNA concentration by using the Qubit dsDNA HS Assay Kit. Ensure a 1/10 dilution is used, as the Qubit reading will be affected by high salt concentration.
22. Store the sample at 4°C until ready to begin the library preparation.
23. Optional Step: Analyse ~100 ng of the final DNA extract on an Agilent Bioanlyser 12,000 bp chip for average fragment size.
Library preparation
Please refer to the Restriction Enzyme Pore-C info sheet for guidance on which library prep kit to choose.
Sequencing experiment
When starting your sequencing experiment in MinKNOW, select the sequencing kit used for your library preparation under Kit Selection.
Results
| Sample | DNA concentration, ng/μl | Total DNA mass, μg |
|---|---|---|
| Arabidopsis thaliana | 24.2 | 3.63 |
Table 1. The yield of non-size selected RE-Pore-C DNA extract using NlaIII restriction enzyme.
Figure 2. Agilent Bioanalyser DNA 12000 trace of non-size selected Arabidopsis thaliana RE-Pore-C DNA extract.
Figure 3. The sequencing and Pore-C output for libraries assessed on GridION Mk1. Libraries were generated as described using Arabidopsis thaliana Pore-C extract prepared with the NlaIII restriction enzyme. The read length distributions and output (Gbases) obtained from the libraries generated are shown in panel A and B, respectively. Panel C displays the Pore-C metrics obtained
Change log
| Version | Change note |
|---|---|
| v6, 12th Februrary 2025 | Removed IGEPAL CA-630 (Sigma, I8896-50ML) from consumables list as not used in the method. Updated "Sequencing experiment" section to indicate selection of sequencing kit used for library preparation in the Kit Selection step in MinKNOW. |
| v5, 02nd December 2024 | Corrected table formatting and clarified step 13 of the "Nuclei isolation: 1.5 hours hands-on time, 1.5 hours procedure" section of the document |
| v4, 24th June 2022 | Corrected the nuclei isolation buffer components |
| v3, 2nd March 2022 | Updated step 10 of the day 3 prep to the correct volume of NaCl and the correct concentration |
| v2, 25th March 2021 | Updated materials to include "ECOSURF™ EH-9 (Sigma, STS0006) or Triton X-100 (Sigma, X100)”. Updated Day 1, step 5 to include ECOSURF™ EH-9. Updated Nuclei isolation buffer table to include ECOSURF™ EH-9. Updated section: Chromatin denaturation, step 4 to include ECOSURF™ EH-9. Updated Proximity ligation B with heat denaturation table to include ECOSURF™ EH-9. |
| v1, 30th July 2020 | Initial protocol publication |






