Ligation sequencing gDNA - Lambda control (Q-SQK-LSK109 with Q-EXP-CTL001) (Q_LCE_revC_26Sep2024)
GridION: Protocol
Ligation sequencing gDNA - Lambda control (Q-SQK-LSK109 with Q-EXP-CTL001) V Q_LCE_revC_26Sep2024
- This protocol uses Lambda control DNA
- High yield
- Library preparation time ~60 minutes
- Fragmentation optional
- No PCR
For Research Use Only
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
Library preparation
- 4. DNA repair and end-prep
- 5. Adapter ligation and clean-up
- 6. Priming and loading the MinION Flow Cell
Sequencing and data analysis
Overview
- This protocol uses Lambda control DNA
- High yield
- Library preparation time ~60 minutes
- Fragmentation optional
- No PCR
For Research Use Only
1. Overview of the protocol
Ligation Sequencing Kit features
This kit is recommended for users who:
- want to optimise their sequencing experiment for throughput
- would like to utilise upstream processes such as size selection, whole genome amplification, or enrichment for long reads
Introduction to the Ligation Sequencing Kit
The Ligation Sequencing Kit is designed to prepare genomic, amplicon, and cDNA, with or without barcoding, for sequencing on Oxford Nanopore devices to produce 1D reads. This kit can be used to prepare libraries from whole genome amplified DNA, starting with as little as 10 pg genomic DNA.
The kit contains an adapter, which must be ligated onto end-repaired and A-tailed fragments. This adapter is loaded with the motor protein that translocates DNA through the nanopore. The workflow for nanopore sequencing consists of steps for template preparation, and then steps required for adapter ligation.
This protocol describes how to carry out sequencing of a Lambda control DNA sample using the Ligation Sequencing Kit (Q-SQK-LSK109) and the Control Expansion (Q-EXP-CTL001).
Steps in the sequencing workflow
Prepare for your experiment
You will need to:
- Ensure you have your sequencing kit, the correct equipment, and third-party reagents
- Library preparation
You will need to:
- Repair the DNA and prepare the DNA ends for adapter attachment
- Attach sequencing adapters, supplied in the kit, to the DNA ends
- Prime the flow cell and load your DNA library into the flow cell
Sequencing and analysis
You will need to:
- Start a sequencing run using the sequencing software, which will collect raw data from the device and convert it into basecalled reads
Compatibility of this protocol
This protocol should only be used in combination with:
- Ligation Sequencing Kit (Q-SQK-LSK109)
- Control Expansion (Q-EXP-CTL001)
- Q-FLO-MIN106D flow cells
2. Equipment and consumables
Materials
- Ligation Sequencing Kit (Q-SQK-LSK109)
- Control Expansion (Q-EXP-CTL001)
- Flow Cell Priming Kit (Q-EXP-FLP002)
Equipment
- Hula mixer (gentle rotator mixer)
- Magnetic separation rack, suitable for 1.5 ml Eppendorf tubes
- Microfuge
- Vortex mixer
- Thermal cycler
- P1000 pipette and tips
- P200 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- P2 pipette and tips
- Ice bucket with ice
- Timer
Optional equipment
- Agilent Bioanalyzer (or equivalent)
- Qubit™ fluorometer (or equivalent for QC check)
- Eppendorf 5424 centrifuge (or equivalent)
For this protocol, you will need the Lambda DNA (LMD) supplied in the Control Expansion pack and we recommend starting with 1 µg (or 100-200 fmol) Lambda DNA.
Ligation Sequencing Kit (Q-SQK-LSK109) contents
Control Expansion (Q-EXP-CTL001) contents

Note: The DNA Control Sample (DCS) is a 3.6 kb standard amplicon mapping the 3' end of the Lambda genome.
Flow Cell Priming Kit (Q-EXP-FLP002) contents

3. Computer requirements and software
GridION IT requirements
The GridION device contains all the hardware required to control up to five sequencing experiments simultaneously and acquire the data. The device is further enhanced with high performance GPU technology for real-time basecalling. Read more in the GridION Q IT Requirements document in the Q system channel.
The sequencing software
The sequencing software controls the GridION, collects sequencing data in real-time and processes it into basecalls. The software can also demultiplex reads by barcode, and basecall/demultiplex data after a sequencing run has completed. You will be using the sequencing software for every assay you run.
For instructions on how to run the sequencing software on the GridION, please refer to the Q-Line sequencing software user guide.
4. DNA repair and end-prep
Materials
- Lambda DNA (LMD)
- DNA Control Sample (DCS)
Consumables
- 0.2 ml thin-walled PCR tubes
- 1.5 ml Eppendorf DNA LoBind tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
- NEBNext® FFPE DNA Repair Mix (NEB, M6630)
- NEBNext® Ultra™ II End Repair/dA-Tailing Module (NEB, E7546)
- Agencourt AMPure XP beads (Beckman Coulter™, A63881)
- Freshly prepared 80% ethanol in nuclease-free water
Equipment
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
- Thermal cycler
- Microfuge
- Hula mixer (gentle rotator mixer)
- Magnetic separation rack
- Ice bucket with ice
Thaw DNA Control Sample (DCS) and Lambda DNA (LMD) at room temperature, spin down, mix gently by pipetting, and place on ice.
Prepare the NEBNext FFPE DNA Repair Mix and NEBNext Ultra II End Repair / dA-tailing Module reagents in accordance with manufacturer’s instructions, and place on ice.
For optimal performance, NEB recommend the following:
Thaw all reagents on ice.
Flick and/or invert the reagent tubes to ensure they are well mixed.
Note: Do not vortex the FFPE DNA Repair Mix or Ultra II End Prep Enzyme Mix.Always spin down tubes before opening for the first time each day.
The Ultra II End Prep Buffer and FFPE DNA Repair Buffer may have a little precipitate. Allow the mixture to come to room temperature and pipette the buffer up and down several times to break up the precipitate, followed by vortexing the tube for 30 seconds to solubilise any precipitate.
Note: It is important the buffers are mixed well by vortexing.The FFPE DNA Repair Buffer may have a yellow tinge and is fine to use if yellow.
In a 0.2 ml thin-walled PCR tube, mix the following:
| Reagent | Volume |
|---|---|
| Nuclease-free water | 27 µl |
| Lambda DNA (50 µg/ml) | 20 µl |
| DNA CS (DCS) | 1 µl |
| NEBNext FFPE DNA Repair Buffer | 3.5 µl |
| NEBNext FFPE DNA Repair Mix | 2 µl |
| Ultra II End-prep reaction buffer | 3.5 µl |
| Ultra II End-prep enzyme mix | 3 µl |
| Total | 60 µl |
Ensure the components are thoroughly mixed by pipetting, and spin down.
Using a thermal cycler, incubate at 20°C for 5 minutes and 65°C for 5 minutes.
AMPure XP bead clean-up
It is recommended that the repaired/end-prepped DNA sample is subjected to the following clean-up with AMPure XP beads. This clean-up can be omitted for simplicity and to reduce library preparation time. However, it has been observed that omission of this clean-up can: reduce subsequent adapter ligation efficiency, increase the prevalence of chimeric reads, and lead to an increase in pores being unavailable for sequencing. If omitting the clean-up step, proceed to the next section.
Resuspend the AMPure XP beads by vortexing.
Transfer the DNA sample to a clean 1.5 ml Eppendorf DNA LoBind tube.
Add 60 µl of resuspended AMPure XP beads to the end-prep reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Prepare 500 μl of fresh 80% ethanol in nuclease-free water.
Spin down the sample and pellet on a magnet until supernatant is clear and colourless. Keep the tube on the magnet, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 200 µl of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 61 µl nuclease-free water. Incubate for 2 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Remove and retain 61 µl of eluate into a clean 1.5 ml Eppendorf DNA LoBind tube.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Take forward the repaired and end-prepped DNA into the adapter ligation step. However, at this point it is also possible to store the sample at 4°C overnight.
5. Adapter ligation and clean-up
Materials
- Adapter Mix (AMX)
- Ligation Buffer (LNB)
- Long Fragment Buffer (LFB)
- S Fragment Buffer (SFB)
- Elution Buffer (EB) from the Ligation Sequencing Kit
Consumables
- NEBNext Quick Ligation Module (NEB, E6056)
- 1.5 ml Eppendorf DNA LoBind tubes
- Agencourt AMPure XP beads (Beckman Coulter™, A63881)
Equipment
- Magnetic separation rack
- Microfuge
- Vortex mixer
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
Although the recommended third-party ligase is supplied with its own buffer, the ligation efficiency of Adapter Mix (AMX) is higher when using Ligation Buffer supplied within the Ligation Sequencing Kit.
Spin down the Adapter Mix (AMX) and Quick T4 Ligase, and place on ice.
Thaw Ligation Buffer (LNB) at room temperature, spin down and mix by pipetting. Due to viscosity, vortexing this buffer is ineffective. Place on ice immediately after thawing and mixing.
Thaw the Elution Buffer (EB) at room temperature and mix by vortexing. Then spin down and place on ice.
Depending on the wash buffer (LFB or SFB) used, the clean-up step after adapter ligation is designed to either enrich for DNA fragments of >3 kb, or purify all fragments equally.
- To enrich for DNA fragments of 3 kb or longer, use Long Fragment Buffer (LFB)
- To retain DNA fragments of all sizes, use Short Fragment Buffer (SFB)
To enrich for DNA fragments of 3 kb or longer, thaw one tube of Long Fragment Buffer (LFB) at room temperature, mix by vortexing, spin down and place on ice.
To retain DNA fragments of all sizes, thaw one tube of Short Fragment Buffer (SFB) at room temperature, mix by vortexing, spin down and place on ice.
In a 1.5 ml Eppendorf DNA LoBind tube, mix in the following order:
Between each addition, pipette mix 10 - 20 times.
| Reagent | Volume |
|---|---|
| DNA sample from the previous step | 60 µl |
| Ligation Buffer (LNB) | 25 µl |
| NEBNext Quick T4 DNA Ligase | 10 µl |
| Adapter Mix (AMX) | 5 µl |
| Total | 100 µl |
Ensure the components are thoroughly mixed by pipetting, and spin down.
Incubate the reaction for 10 minutes at room temperature.
If you have omitted the AMPure purification step after DNA repair and end-prep, do not incubate the reaction for longer than 10 minutes.
Resuspend the AMPure XP beads by vortexing.
Add 40 µl of resuspended AMPure XP beads to the reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Spin down the sample and pellet on a magnet. Keep the tube on the magnet, and pipette off the supernatant when clear and colourless.
Wash the beads by adding either 250 μl Long Fragment Buffer (LFB) or 250 μl Short Fragment Buffer (SFB). Flick the beads to resuspend, spin down, then return the tube to the magnetic rack and allow the beads to pellet. Remove the supernatant using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual supernatant. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 15 µl Elution Buffer (EB). Spin down and incubate for 10 minutes at room temperature. For high molecular weight DNA, incubating at 37°C can improve the recovery of long fragments.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Remove and retain 15 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads
Quantify 1 µl of eluted sample using a Qubit fluorometer.
The prepared library is used for loading into the flow cell. Store the library on ice or at 4°C until ready to load.
We recommend loading 5–50 fmol of this final prepared library onto R9.4.1 flow cells. For R10.3 flow cells, we recommend loading 25-75 fmol.
Loading more than 50 fmol of DNA can have a detrimental effect on throughput. Dilute the library in Elution Buffer if required. If you are using the Flongle for sample prep development, we recommend loading 3-20 fmol instead.
If you are using Flongle, please follow the flow cell priming and loading instructions as described in the Flongle version of this protocol, "Loading the Flongle flow cell" section.
6. Priming and loading the MinION Flow Cell
Materials
- Flush Tether (FLT)
- Flush Buffer (FB)
- Sequencing Buffer (SQB)
- Loading Beads (LB)
Consumables
- 1.5 ml Eppendorf DNA LoBind tubes
- Nuclease-free water (e.g. Thermo Scientific, AM9937)
Equipment
- MinION Flow Cell
- P1000 pipette and tips
- P100 pipette and tips
- P20 pipette and tips
- P10 pipette and tips
Thaw the Sequencing Buffer (SQB), Loading Beads (LB), Flush Tether (FLT) and one tube of Flush Buffer (FB) at room temperature before mixing the reagents by vortexing, and spin down at room temperature.
To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Slide open the GridION lid and insert the flow cell.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Slide the flow cell priming port cover clockwise to open the priming port.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
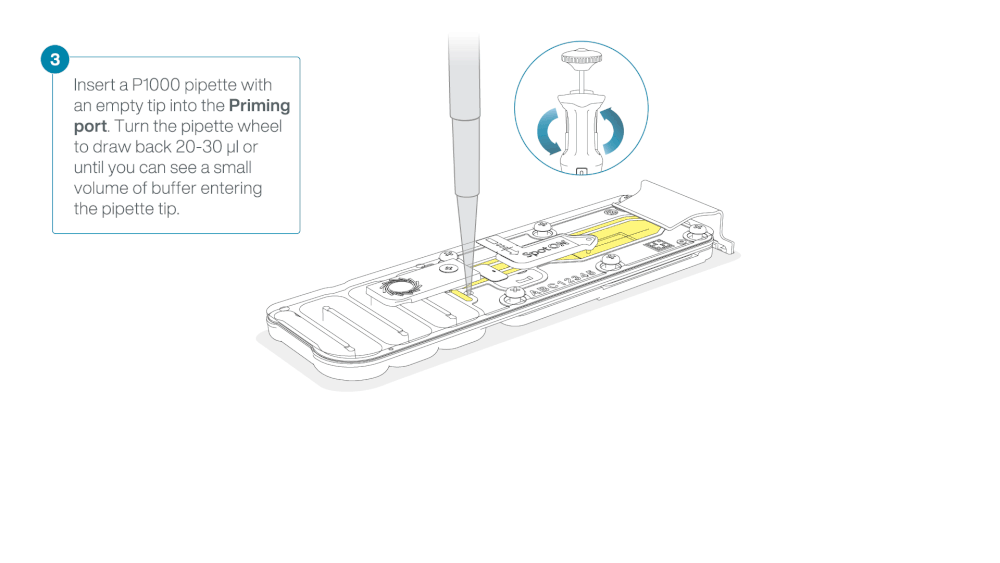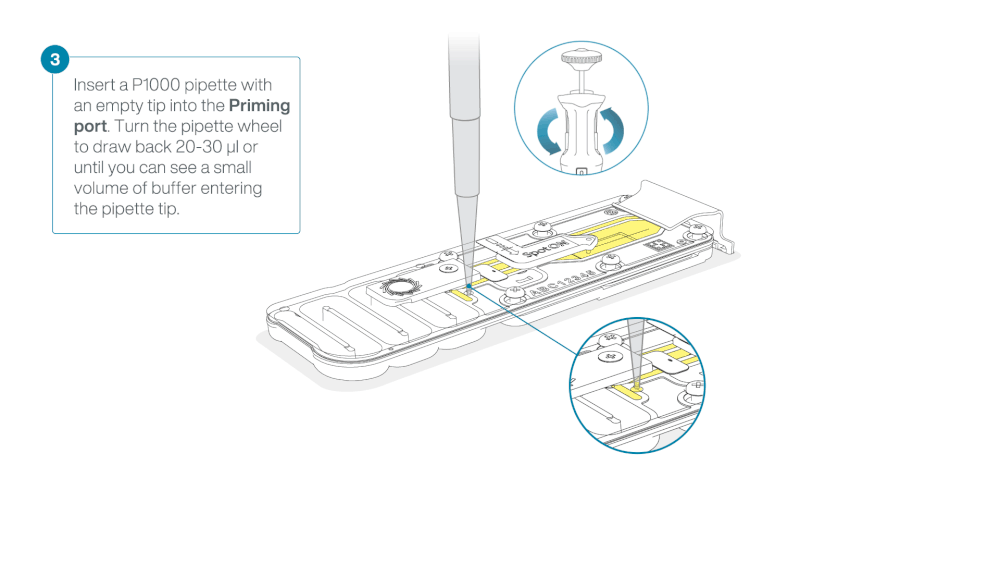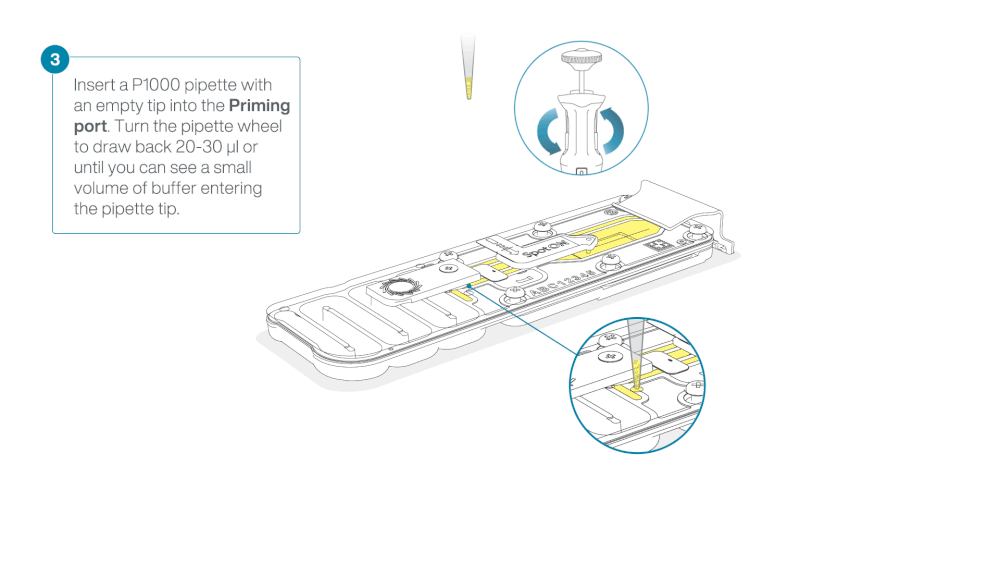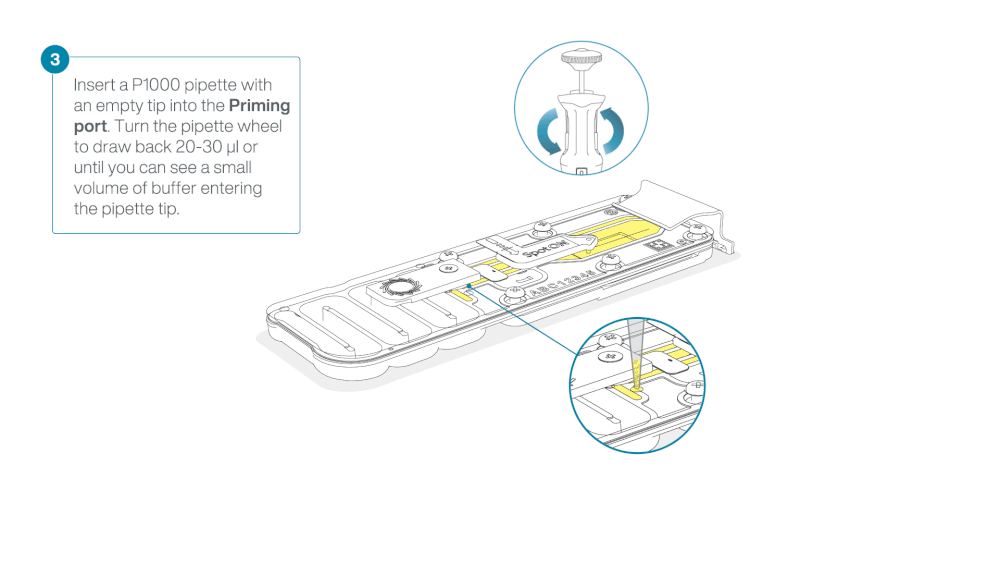
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 µl, to draw back 20-30 µl, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the Loading Beads (LB) by pipetting.
The Loading Beads (LB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SQB) | 37.5 µl |
| Loading Beads (LB), mixed immediately before use | 25.5 µl |
| DNA library | 12 µl |
| Total | 75 µl |
Note: Load the library onto the flow cell immediately after adding the Sequencing Buffer (SQB) and Loading Beads (LB) because the fuel in the buffer will start to be consumed by the adapter.
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the flow cell priming port and close the GridION lid.
7. Data acquisition and basecalling
How to start sequencing
Follow the instructions in the Q-Line sequencing software user guide beginning from the "Set up and run an assay" section.







