Human variation sequencing from 30kb extracted cell line samples using SQK-LSK114 (GDH_9174_v114_revT_11Sep2025)
PromethION: Protocol
V GDH_9174_v114_revT_11Sep2025
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
Sample preparation
- 3. Human cell line DNA extraction using the QIAGEN Puregene Cell Kit
- 4. Size selection of gDNA using SFE Buffer
- 5. gDNA fragmentation
Library preparation
- 6. DNA repair and end-prep
- 7. Adapter ligation and clean-up
- 8. Priming and loading the PromethION Flow Cell
- 9. Washing and reloading a PromethION Flow Cell
Sequencing and data analysis
Troubleshooting
1. Overview of the protocol
Introduction to the human variation sequencing from 30kb extracted cell line samples using SQK-LSK114 protocol
This protocol describes an end-to-end process to prepare and sequence gDNA from cultured cell samples and analyse them using the “Human variant workflow” in EPI2ME. The identification of structural variants (SVs) and single nucleotide variants (SNVs) play a pivotal role in our understanding of genetic diversity, disease mechanisms, and evolutionary biology. The protocol aims to produce libraries with a read N50 ~30 kb and generate ~30-40x coverage of the genome, to provide robust calling of small and large variants, as well as methylation and phasing information.
Briefly, genomic DNA is extracted from 5 million cultured cells using the QIAGEN Puregene Cell Kit. DNA is size selected using the Short Fragment Eliminator Kit (EXP-SFE001) and then sheared with Megaruptor® 3† (Diagenode). Libraries are prepared with the Ligation Sequencing Kit V14 (SQK-LSK114).
Libraries are sequenced on PromethION and we detail instructions for washing and reloading the flow cell twice to maximise data output using the Flow Cell Wash Kit (EXP-WSH004) and Sequencing Auxiliary Vials V14 (EXP-AUX003). Data is basecalled and aligned by MinKNOW and the aligned BAM output data is analysed using the wf-human-variation workflow which uses Sniffles2, Clair3 and modbam2bed software to call structural variants (SVs), single nucleotide polymorphisms (SNPs) and for reporting DNA methylation.
†Note: Users who do not have access to the Megaruptor and wish to omit this step are likely to observe a drop in coverage from ~40x to ~30x.
Steps in the sequencing workflow:
Prepare for your experiment
You will need to:
- Extract your input sample (cells).
- Ensure you have your sequencing kit, the correct equipment, and third-party reagents.
- Download the software for acquiring and analysing your data.
- Check your flow cell to ensure it has enough pores for a good sequencing run.
Sample preparation
Using the outlined extraction method, extract the gDNA from your cells, perform size selection using the Short Fragment Eliminator Kit (EXP-SFE001), and fragment your gDNA using the Megaruptor.
Check the length, quantity and purity of your extracted material. The quality checks performed during the protocol are essential in ensuring experimental success.
Library preparation
The Table below is an overview of the steps required in the library preparation, including timings and optional stopping points.
| Library preparation | Process | Time | Stop option |
|---|---|---|---|
| DNA repair and end-prep | Repair and prepare the DNA ends for adapter attachment | 35 minutes | 4°C overnight |
| Adapter ligation and clean-up | Attach the sequencing adapters to the DNA ends | 55 minutes | 4°C short-term storage or for repeated use, such as re-loading your flow cell -80°C for single-use, long-term storage. We strongly recommend sequencing your library as soon as it is adapted. |
| Priming and loading the flow cell | Prime the flow cell and load the prepared library for sequencing | 10 minutes | |
| Washing and reloading the flow cell (x2) | Pause your sequencing run. Wash your flow cell with nuclease to remove the previous library load and unblock pores. Prime the flow cell and reload the prepared library to continue sequencing | 60 minutes (x2) |
Sequencing and analysis
You will need to:
- Start a sequencing run using the MinKNOW software which will collect raw data from the device and convert it into basecalled reads.
- Analyse data using the wf-human-variation workflow.
Compatibility of this protocol
This protocol should only be used in combination with:
- Ligation Sequencing Kit V14 (SQK-LSK114)
- R10.4.1 PromethION Flow Cells (FLO-PRO114M)
- Short Fragment Eliminator Expansion (EXP-SFE001)
- Flow Cell Wash Kit (EXP-WSH004)
- Sequencing Auxiliary Vials V14 (EXP-AUX003)
- PromethION 24/48 device - PromethION IT requirements document
- PromethION 2 Solo device - PromethION 2 Solo IT requirements document
2. Equipment and consumables
材料
- (用于提取)5 × 10⁶ 个细胞(例如细胞培养物或组织样本)
- (FOR LIBRARY PREPARATION) 3 µg of SFE size selected and Megaruptor fragmented gDNA
- Short Fragment Eliminator (EXP-SFE001) kit
- 连接测序试剂盒V14(SQK-LSK114)
- 测序芯片清洗剂盒(EXP-WSH004)
- 测序辅助扩展包 V14(EXP-AUX003)
耗材
- PromethION 测序芯片
- 供 Oxford Nanopore Technologies® 连接测序使用的 NEBNext® 配套模块 v2(NEB, E7672S 或 E7672L)
- Puregene 细胞试剂盒(QIAGEN,158043)
- Megaruptor 3 Shearing Kit (Diagenode, E07010003)
- 无核酸酶水(如ThermoFisher,AM9937)
- 15 ml Falcon离心管
- 2 ml Eppendorf DNA LoBind 离心管
- 1.5 ml Eppendorf DNA LoBind 离心管
- 0.2 ml 薄壁PCR管
- 新制备的 80% 乙醇(用无核酸酶水配制)
- 磷酸盐缓冲盐溶液(PBS),pH 7.4(ThermoFisher,10010023)
- 异丙醇,100%(Fisher, 10723124)
- TE 缓冲液(10 mM Tris-HCl、1 mM EDTA、pH 8.0)(Fisher scientific,10224683)
- 接种环或一次性镊子
- Qubit™ 分析管(Invitrogen, Q32856)
- Qubit dsDNA BR Assay(双链DNA宽范围检测)试剂盒(ThermoFisher ,Q32850)
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
- Agilent 基因组 DNA 165 kb 分析试剂盒(Agilent,FP-1002-0275)
仪器
- PromethION 测序设备
- PromethION 测序芯片遮光片
- Hula混匀仪(低速旋转式混匀仪)
- 适用于1.5ml Eppendorf 离心管的磁力架
- 迷你离心机
- 涡旋混匀仪
- 热循环仪
- 适用于 15 ml Falcon 管的离心机及转子
- 设定为 37°C 和 50°C 的培养箱或水浴锅
- Megaruptor 3 (Diagenode, B06010003)
- 宽口移液枪头
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
- 盛有冰的冰桶
- 计时器
- Heating block
- 盛有冰的冰桶
- Agilent Femto Pulse 系统(或用于读长质控的等效仪器)
- Qubit™ 荧光计(或用于质控检测的等效仪器)
可选仪器
- Shaking heat block
The above list of materials, consumables, and equipment is for the extraction method in the sample preparation section, as well as the library preparation section of the protocol. If you have pre-extracted sample(s), you will only require the materials for the library preparation section of this protocol.
For the library preparation protocol, you will need 3 µg of genomic DNA with an N50 of 30 kb.
For this end-to-end workflow, we recommend extracting high molecular weight gDNA from 5x10⁶ cells (e.g. cell culture or tissue sample) using the QIAGEN Puregene Cell Kit in the Sample Preparation step.
Other extraction protocols are available but have not been tested by Oxford Nanopore Technologies.
We recommend using wide-bore pipette tips during sample and library preparation to avoid mechanical shearing of your sample.
To preserve longer fragment DNA, mix slowly and more gently. However, it is important to ensure reagents are thoroughly mixed with the DNA. Insufficient mixing will lead to reduced read length and output.
Input DNA
How to QC your input DNA
It is important that the input DNA meets the quantity and quality requirements. Using too little or too much DNA, or DNA of poor quality (e.g. highly fragmented or containing RNA or chemical contaminants) can affect your library preparation.
For instructions on how to perform quality control of your DNA sample, please read the Input DNA/RNA QC protocol.
Chemical contaminants
Depending on how the DNA is extracted from the raw sample, certain chemical contaminants may remain in the purified DNA, which can affect library preparation efficiency and sequencing quality. Read more about contaminants on the Contaminants page of the Community.
Eppendorf tube orientation in centrifuge
For all centrifugation steps, ensure that tubes are loaded into the centrifuge with the hinge side of the tube facing outwards. This will assist in visual identification of the pellet.
Ensure gentle handling when removing the tubes from the centrifuge to avoid dislodging the pellet.

We recommend using the NEBNext® Companion Module v2 for Oxford Nanopore Technologies® Ligation Sequencing (NEB, E7672S or E7672L), which contains all the NEB reagents needed for use with the Ligation Sequencing Kit.
The previous version, NEBNext® Companion Module for Oxford Nanopore Technologies® Ligation Sequencing (NEB, E7180S or E7180L) is also compatible, but the recommended v2 module offers more efficient dA-tailing and ligation.
Third-party reagents
We have validated and recommend the use of all the third-party reagents used in this protocol. Alternatives have not been tested by Oxford Nanopore Technologies.
For all third-party reagents, we recommend following the manufacturer's instructions to prepare the reagents for use.
Check your flow cell
We highly recommend that you check the number of pores in your flow cell prior to starting a sequencing experiment. This should be done within 12 weeks of purchasing your PromethION Flow Cells. Oxford Nanopore Technologies will replace any unused flow cell with fewer than the number of pores listed in the Table below, when the result is reported within two days of performing the flow cell check, and when the storage recommendations have been followed. To do the flow cell check, please follow the instructions in the Flow Cell Check document.
| Flow cell | Minimum number of active pores covered by warranty |
|---|---|
| PromethION Flow Cell | 5000 |
We strongly recommend using the Ligation Buffer (LNB) supplied in the Ligation Sequencing Kit V14 rather than any third-party ligase buffers to ensure high ligation efficiency of the Ligation Adapter (LA).
Ligation Adapter (LA) included in this kit and protocol is not interchangeable with other sequencing adapters.
Ligation Sequencing Kit V14 (SQK-LSK114) contents
Note: This product contains AMPure XP reagent manufactured by Beckman Coulter, Inc. and can be stored at -20°C with the kit without detriment to reagent stability.
Note: The DNA Control Sample (DCS) is a 3.6 kb standard amplicon mapping the 3' end of the Lambda genome.
3. Human cell line DNA extraction using the QIAGEN Puregene Cell Kit
材料
- 5x10⁶ cells(例如细胞培养物或组织样本)
耗材
- Puregene 细胞试剂盒(QIAGEN,158043)
- Freshly prepared cold 80% ethanol in nuclease-free water
- TE 缓冲液(10 mM Tris-HCl、1 mM EDTA、pH 8.0)(Fisher scientific,10224683)
- 磷酸盐缓冲盐溶液(PBS),pH 7.4(ThermoFisher,10010023)
- 异丙醇,100%(Fisher, 10723124)
- 15 ml Falcon离心管
- 2 ml Eppendorf DNA LoBind 离心管
- 1.5 ml Eppendorf DNA LoBind 离心管
- 接种环或一次性镊子
- Qubit dsDNA BR Assay(双链DNA宽范围检测)试剂盒(ThermoFisher ,Q32850)
- Qubit™ 分析管(Invitrogen, Q32856)
仪器
- 设定为 37°C 和 50°C 的培养箱或水浴锅
- 适用于 15 ml Falcon 管的离心机及转子
- 涡旋混匀仪
- 宽口移液枪头
- P1000 移液枪和枪头
- P200 移液枪和枪头
- 盛有冰的冰桶
- Qubit™ 荧光计(或用于质控检测的等效仪器)
We recommend using wide-bore pipette tips during sample and library preparation to avoid mechanical shearing of your sample.
To preseve longer DNA, mix slower and more gently. However, it is important to ensure reagents are thoroughly mixed with the DNA. Insufficient mixing will lead to reduced read length and output.
Prepare a 1.5 ml Eppendorf DNA LoBind tube with approximately 1 ml of 80% ethanol and store on ice to cool.
In a 15 ml falcon tube, harvest and pellet 5 x 10^6 cells by centrifugation at 300 x g for 3 minutes. If any liquid remains associated with the pellet, spin down the cells again, then aspirate and discard the remaining supernatant.
Note: Reuqired cell numbers may vary depending on your sample type.
Add 200 µl of 1x PBS to pelleted cells.
Centrifuge at 300 x g for 3 minutes.
Without disturbing the pellet, aspirate and discard the supernatant.
Add 200 µl of 1x PBS to the pelleted cells, and gently flick the tube or pipette mix using a wide bore tip to resuspend the pellet.
The pellet should be completely dispersed after, and this greatly facilitates the cell lysis in the next step.
Add 3 ml of Cell Lysis Solution.
Note: Pipette mix gently 10-15 times to lyse the cells and homogenise the solution until no clumps remain. Ensure that the solution is homogenous.
Incubate the sample at 37°C for 30 minutes.
Note: Ensure the solution is homogenous by the end of the incubation, and no clumps should remain.
If necessary, you can mix the reaction by pipette mixing with a wide bore pipette tip or gently inverting the tube to assist with homogenisation.
Add 5 µl of RNase A solution to the sample tube, and invert the tube 20 times to mix.
Incubate the sample tube for 15 minutes at 37°C, then transfer the tube to ice and incubate for 3 minutes to quickly cool the sample.
Add 1000 µl of the Protein Precipitation Solution to the lysed cells and mix by vortexing for two pulses of 5 seconds at maximum speed.
Centrifuge the sample at 2000 x g for 10 minutes.
The precipitated protein should form a tight, white pellet.
If the protein pellet is not tight, incubate on ice for 5 minutes and repeat the centrifugation.
Add 3 ml of isopropanol into a clean 15 ml falcon tube.
Carefully pour the supernatant from your sample tube into the 15 ml falcon tube containing the isopropanol without disturbing the pellet.
Ensure that the protein pellet is not dislodged during pouring.
Alternatively, the supernatant can also be transferred by pipetting, ensuring the protein pellet remains undisturbed and is intact when transferring.
Note: If at any point the protein pellet is disturbed, repeat the 10 minute at 2000 x g centrifugation step. Ensure only the clear supernatant is transferred to avoid protein contamination in the final elute.
Mix by gently inverting the tube 50 times until the DNA is visible as threads or a clump.
Centrifuge the falcon tube for 3 minutes at 2000 x g.
The DNA may be visible as a small white pellet at the bottom of the tube.
Carefully discard the supernatant and drain the tube by inverting on a clean piece of absorbent paper. Ensure the DNA pellet is undisturbed and remains in the tube.
Note: The supernatant can be removed by pipetting or by pouring the volume out on to an absorbent material.
Take care as the pellet might be loose and easily dislodged.
Add 300 μl of ice-cold freshly-prepared 80% ethanol to the sample tube. Gently invert the tube several times to wash the DNA pellet.
Transfer the pellet and the full 300 μl volume of ethanol into a new 1.5 ml Eppendorf tube.
Centrifuge the sample tube for 1 minute at 2000 x g.
Carefully discard the supernatant and drain the tube by inverting on a clean piece of absorbent paper. Ensure the DNA pellet is undisturbed and remains in the tube.
Note: The supernatant can be removed by pipetting or by pouring the volume out on to an absorbent material.
Take care as the pellet might be loose and easily dislodged.
Leave the lid off the sample tube and air dry the pellet for 1 min.
Note: Avoid over-drying the pellet, ensure it is not dried to the point of cracking.
Add 200 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) to the tube containing the sample pellet. Gently resuspend the pellet by flicking.
Incubate the tube for 2 hours at 50°C, occasionally pipette mixing the whole volume tube contents (200 μl) with a wide-bore pipette tip.
Note: The DNA pellet may take some time to solubilise. Please ensure the solution is homogenous before quantifying.
Optional: Alternatively, this incubation can be performed at room temperature overnight.
Quantify your sample three times using the Qubit dsDNA BR Assay Kit. Ensure the replicate Qubit measurements are consistent before continuing to the next step.
Note: Approximately 20 - 35 µg of gDNA is expected following extraction from 5 x 10⁶ cultured cells.
Expected Qubit measurements of 200–350 ng/μl.
If your Qubit measurements are not consistent, this could indicate that the DNA has not been homogeneously resuspended.
If this occurs, we recommend increasing the incubation time, allowing more time for the DNA pellet to solubilise.
Note: The elution can be aided by incubating at 50°C on a thermomixer with gentle agitation 300 RPM. Alternatively an end over end rotating mixer can be used.
Your extracted gDNA can also be analysed using Femto Pulse (Agilent) to check the size and quality.
Example fragment length profile of gDNA extracted from cells using the Puregene Cell Kit.
Take your extracted gDNA forward into the size selection of gDNA step of this protocol. Alternatively, your sample can be stored at 4°C overnight.
4. Size selection of gDNA using SFE Buffer
材料
- 10 µg extracted human gDNA
- SFB Expansion (EXP-SFE001)
耗材
- 无核酸酶水(如ThermoFisher,AM9937)
- 新制备的 80% 乙醇(用无核酸酶水配制)
- Qubit dsDNA BR Assay(双链DNA宽范围检测)试剂盒(ThermoFisher ,Q32850)
- Qubit™ 分析管(Invitrogen, Q32856)
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- 离心机
- Heating block
- 涡旋混匀仪
- 迷你离心机
- Qubit™ 荧光计(或用于质控检测的等效仪器)
- 盛有冰的冰桶
- 计时器
- 宽口移液枪头
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
可选仪器
- Agilent Femto Pulse 系统(或用于读长质控的等效仪器)
We recommend substituting nuclease-free water for TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) if the library is going to be stored over a long-term period.
Ensure the SFE buffer is at room temperature. Pipette mix the buffer thoroughly, at least 10-15 times, using a wide bore pipette tip
In a 1.5 ml Eppendorf DNA LoBind tube, prepare 10 µg DNA in 100 µl of nuclease-free water to a final concentration of ~100 ng/μl.
The final concentration of gDNA should not exceed ~150 ng/μl.
Add an equal volume (100 µl) of Short Fragment Eliminator (SFE) buffer to the DNA sample and slowly and gently pipette mix 10-15 times using a wide-bore pipette tip.
Place the tube in the centrifuge with the tube hinge facing outward, and add the appropriate tubes to balance the centrifuge.
Ensure proper tube orientation before starting and that the centrifuge is at the correct temperature prior use.
Centrifuge the sample at 10,000 x g at 22°C for 30 minutes.
Note: Ensure that the centrifuge is at the correct temperature prior use.
The pellet may not be visible but it will be located on the side of the tube that was facing outwards during centrifugation. We recommend not removing the full volume of the supernatant and leaving ~15–20 µl as this will be washed out in subsequent steps.
Using a narrow bore pipette tip, slowly and carefully remove most of the supernatant (leaving ~ 15-20 μl behind) by aspirating from the opposite side of the tube from the location of the pellet, taking care not to disturb the pellet.
Note: To protect against loss of DNA by inadvertent aspiration of pelleted DNA, keep the volume of the aspirated supernatant and store it in a separate 1.5 ml Eppendorf tube.
Ensure you label your tube with the saved supernatent for tracking.
To the ~15–20 μl of the volume left behind in your sample tube, slowly add 200 μl of freshly prepared 80% ethanol to the tube, without disturbing the pellet (we recommend dispensing on the opposite side of the tube as your pellet). Centrifuge the sample at 10,000 x g for 3 minutes ensuring the same tube orientation used in for the previous centrifuge step (hinge facing outwards). Using a narrow bore tip, slowly and gently aspirate the supernatant from the opposite side of the tube, taking care not to disturb the pellet.
Note: After the first ethanol wash, the pellet may become more visible (as a white pellet at the bottom of the hinge-side of the tube):
- If the pellet is visible, then carefully discard as much of the ethanol as you can.
- If the pellet is not visible, leave behind ~20 μl volume of supernatant to avoid inadvertently aspirating or disturbing the pellet.
Note: To protect against loss of DNA by inadvertent aspiration of pelleted DNA, the aspirated supernatant should be stored in a separate 1.5 ml Eppendorf tube, labelled accordingly for tracking and stored in the fridge.
Repeat the previous step.
Note: After the second ethanol wash, the pellet should be clearly visible in most cases and the residual ethanol can be carefully discarded using a 10 μl pipette, taking care not to disturb the pellet.
Note: To protect against loss of DNA by inadvertent aspiration of pellet DNA, the aspirated supernatant should be stored in a separate 1.5 ml Eppendorf tube, labelled accordingly for tracking and stored in the fridge.
Add 100 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) to the DNA pellet and pipette mix using a wide-bore tip.
Incubate the tube in a heat block at 37°C for 30 minutes. Gently agitate the solution by flicking every 5 minutes to aid with resuspension.
Alternatively, a thermomixer set to at 300 rpm for 30 minutes can facilitate faster elution of DNA.
Optional: If necessary, the pellet can be left overnight to elute at room temperature.
Gently mix the tube contents by pipetting up and down using a wide-bore tip.
Quantify your sample three times using the Qubit dsDNA BR Assay Kit. Ensure the replicate Qubit measurements are consistent before continuing to the next step.
The readings should be consistent. If they are not, consider:
- additional mixing steps/agitation steps with a wide-bore tip.
- additional mixing steps/agitation steps in a thermomixer set to 300 rpm.
- increasing elution time to facilitate homogenisation.
Note: Approximately 3–6 µg of gDNA is expected following SFE size selection.
Expected Qubit measurements of ~33.33–66.67 ng/μl.
If your Qubit measurements are not consistent, this could indicate that the DNA has not been homogeneously resuspended.
If this occurs, we recommend increasing the elution time and incubating the DNA at 50°C to aid with resuspension of the DNA pellet.
Ensure you have sufficient sample recovery to proceed with the method:
If sample recovery is >3 μg:
Proceed to gDNA Fragmentation using the Megaruptor.
If sample recovery is <3 μg:
The first stored supernatant can be re-processed to yield additional DNA. This can be achieved by performing an additional 10,000 x g centrifugation at room temperature for 30 minutes. Then proceed with the ethanol and wash steps as indicated in method.
Following re-processing of the supernatant:
- We recommend to QC on the femtopulse before combining the two elutes.
- The combined elutes should now yield >3 ug. Proceed to gDNA Fragmentation using the Megaruptor.
Your SFE size selected gDNA can also be analysed using Femto Pulse (Agilent) to check the size and quality.

Example fragment length profile of extracted gDNA, size selected using SFE buffer.
Take your SFE size selected gDNA forward into the fragmentation step of this protocol. Alternatively, your sample can be stored at 4°C overnight.
5. gDNA fragmentation
材料
- 3 µg of SFE size selected gDNA
耗材
- Megaruptor 3 Shearing Kit (Diagenode, E07010003)
- TE 缓冲液(10 mM Tris-HCl、1 mM EDTA、pH 8.0)(Fisher scientific,10224683)
- Agilent 基因组 DNA 165 kb 分析试剂盒(Agilent,FP-1002-0275)
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- Megaruptor 3 (Diagenode, B06010003)
- 迷你离心机
- Agilent Femto Pulse 系统(或用于读长质控的等效仪器)
- 盛有冰的冰桶
- 计时器
- 宽口移液枪头
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
Prepare the DNA in nuclease-free water:
Transfer 3 µg of of SFE size selected gDNA into a clean Megaruptor 3 shearing tube.
Adjust the volume to 90 μl with with 10mM Tris 1mM EDTA pH8.
Mix thoroughly by pipetting up and down using a wide-bore pipette tip.
Spin down briefly in a microfuge.
Transfer the sample tube to the Megaruptor 3, ensuring the instrument is appropriately balanced according to the manufacturers instructions.
Note: Ensure the Megaruptor 3 Hydropore-Syringes are screwed tight before inserting into the Megaruptor.
Ensure no bubbles are present in the sample, and visually confirm that the syringe is immersed 2/3rd of the way into the sample volume.
Setup the shearing parameters on the Megaruptor 3 device as follows:
| Megaruptor 3 setting | |
|---|---|
| Shearing speed | 25 |
| Volume | 90 μl |
| Concentration | 33 ng/ul |
Begin the shearing of DNA using the Megaruptor 3.
Quantify your sample using the Qubit dsDNA BR Assay Kit.
Note: Approximately 3 µg of gDNA is expected following shearing.
Expected Qubit measurements of ~33.33 ng/μl.
Assess the fragmented gDNA for fragment size using Femto Pulse (Agilent).
The SFE size selection removes short DNA fragments from the sample, whilst Megaruptor fragmentation reduces the fragment length profile to a size range between 10 kb and 80 kb, centred around approximately 30 kb.
Overlay of fragment length profiles of a) gDNA extracted using the Puregene cell Kit, b) gDNA extracted and size selected using SFE buffer. c) gDNA extracted, size selected and fragmented using Megaruptor 3 (MR).
Take your SFE size selected and Megaruptor fragmented gDNA forward into the library preparation section of this protocol. Alternatively, your sample can be stored at 4°C overnight.
6. DNA repair and end-prep
材料
- 3 µg of SFE size selected and Megaruptor fragmented gDNA
- AMPure XP 磁珠(AXP)
耗材
- NEBNext®配套模块v2(NEB,E7672S或E7672L)中的NEBNext® FFPE DNA修复混合液
- NEBNext®配套模块v2(NEB,E7672S或E7672L)中的NEBNext® FFPE DNA修复缓冲液v2
- NEBNext®配套模块v2(NEB,E7672S或E7672L)中的NEBNext® Ultra II 末端修复酶混合物
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
- 无核酸酶水(如ThermoFisher,AM9937)
- 新制备的 80% 乙醇(用无核酸酶水配制)
- Qubit™ 分析管(Invitrogen, Q32856)
- 0.2 ml 薄壁PCR管
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- P1000 移液枪和枪头
- P100 移液枪和枪头
- P10 移液枪和枪头
- 迷你离心机
- 热循环仪
- Hula混匀仪(低速旋转式混匀仪)
- 磁力架
- 盛有冰的冰桶
可选仪器
- Qubit™ 荧光计(或用于质控检测的等效仪器)
Check your flow cell.
We recommend performing a flow cell check before starting your library prep to ensure you have a flow cell with enough pores for a good sequencing run.
See the flow cell check document for more information.
Prepare the NEB reagents in accordance with manufacturer’s instructions, and place on ice.
For optimal performance, NEB recommend the following:
Thaw all reagents on ice.
Flick and/or invert the reagent tubes to ensure they are well mixed.
Note: Do not vortex the FFPE DNA Repair Mix or Ultra II End Prep Enzyme Mix.Always spin down tubes before opening for the first time each day.
Vortex the FFPE DNA Repair Buffer v2 to ensure it is well mixed.
Note: This buffer may contain a white precipitate. If this occurs, allow the mixture to come to room temperature and pipette the buffer several times to break up the precipitate, followed by a quick vortex to mix.The FFPE DNA Repair Buffer v2 may have a yellow tinge and is fine to use if yellow.
Prepare the DNA in nuclease-free water:
Transfer 3 µg of SFE size selected and Megaruptor fragmented gDNA from the sample extraction into a 0.2 ml thin-walled PCR tube.
Adjust the volume to 80 μl with nuclease-free water.
Mix thoroughly by pipetting up and down, or by flicking the tube.
Spin down briefly in a microfuge.
In the 0.2 ml thin-walled PCR tube containing your gDNA, mix the following:
| Reagent | Volume |
|---|---|
| DNA from the previous step | 80 µl |
| NEBNext FFPE DNA Repair Buffer v2 | 11.7 µl |
| NEBNext FFPE DNA Repair Mix | 3.3 µl |
| Ultra II End-prep Enzyme Mix | 5 µl |
| Total | 100 µl |
Thoroughly mix the reaction by gently pipetting and briefly spinning down.
Using a thermal cycler, incubate the reaction at 20°C for 5 minutes, then 65°C for 5 minutes and hold at 4°C.
Resuspend the AMPure XP Beads (AXP) by vortexing.
Transfer the DNA sample to a clean 1.5 ml Eppendorf DNA LoBind tube.
Add 100 µl of resuspended the AMPure XP Beads (AXP) to the end-prep reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Prepare 600 µl of fresh 80% ethanol in nuclease-free water.
Spin down the sample and pellet on a magnet for 10 minutes until supernatant is clear and colourless. Keep the tube on the magnet, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 250 µl of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 61 µl nuclease-free water by gently pipetting up and down or by flicking the tube. Incubate for 2 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Remove and retain 61 µl of eluate into a clean 1.5 ml Eppendorf DNA LoBind tube.
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Note: You should expect to recover between 1500–2500 ng.
Take forward the repaired and end-prepped DNA into the adapter ligation step. However, at this point it is also possible to store the sample at 4°C overnight.
7. Adapter ligation and clean-up
材料
- 连接接头(LA)
- 连接测序试剂盒内的连接缓冲液(LNB)
- 长片段缓冲液(LFB)
- AMPure XP 磁珠(AXP)
- 洗脱缓冲液(EB)
耗材
- 耐盐T4 DNA连接酶(NEB, M0467)
- 1.5 ml Eppendorf DNA LoBind 离心管
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
- Qubit™ 分析管(Invitrogen, Q32856)
仪器
- 磁力架
- 迷你离心机
- 涡旋混匀仪
- P1000 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- Qubit™ 荧光计(或用于质控检测的等效仪器)
Although third-party ligase products may be supplied with their own buffer, the ligation efficiency of the Ligation Adapter (LA) is higher when using the Ligation Buffer (LNB) supplied in the Ligation Sequencing Kit.
Spin down the Ligation Adapter (LA) and Salt-T4 DNA Ligase, and place on ice.
Thaw Ligation Buffer (LNB) at room temperature, spin down and mix by pipetting. Due to viscosity, vortexing this buffer is ineffective. Place on ice immediately after thawing and mixing.
Thaw the Elution Buffer (EB) at room temperature and mix by vortexing. Then spin down and place on ice.
Thaw the Long Fragment Buffer (LFB) at room temperature and mix by vortexing. Then spin down and place on ice.
In a 1.5 ml Eppendorf DNA LoBind tube, mix in the following order:
Between each addition, pipette mix 10–20 times.
| Reagent | Volume |
|---|---|
| DNA sample from the previous step | 60 µl |
| Ligation Buffer (LNB) | 25 µl |
| Salt-T4 DNA Ligase | 10 µl |
| Ligation Adapter (LA) | 5 µl |
| Total | 100 µl |
Thoroughly mix the reaction by gently pipetting and briefly spinning down.
Incubate the reaction for 10 minutes at room temperature.
Resuspend the AMPure XP Beads (AXP) by vortexing.
Add 40 µl of resuspended AMPure XP Beads (AXP) to the reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Spin down the sample and pellet on a magnet. Keep the tube on the magnet, and pipette off the supernatant when clear and colourless.
Wash the beads by adding 250 μl Long Fragment Buffer (LFB). Flick the beads to resuspend, spin down, then return the tube to the magnetic rack and allow the beads to pellet for at least 5 minutes. Remove the supernatant using a pipette and discard.
Note: Take care when removing the supernatant, the viscosity of the buffer can contribute to loss of beads from the pellet.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual supernatant. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 97 µl Elution Buffer (EB). Spin down and incubate for 10 minutes at 37°C.
Pellet the beads on a magnet for 10 minutes, until the eluate is clear and colourless.
Remove and retain 97 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Note: You should expect to recover 1200–1800 ng of adapter ligated library in a volume of 96 µl.
The prepared library is used for loading into the flow cell. Store the library on ice or at 4°C until ready to load.
Library storage recommendations
We recommend storing libraries in Eppendorf DNA LoBind tubes at 4°C for short-term storage or repeated use, for example, re-loading flow cells between washes. For single use and long-term storage of more than 3 months, we recommend storing libraries at -80°C in Eppendorf DNA LoBind tubes.
8. Priming and loading the PromethION Flow Cell
材料
- 测序缓冲液(SB)
- 文库颗粒(LIB)
- 测序芯片系绳(FCT)
- 测序芯片冲洗液(FCF)
耗材
- PromethION 测序芯片
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- PromethION 2 Solo 测序设备
- PromethION 测序设备
- PromethION 测序芯片遮光片
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P20 移液枪和枪头
This kit is only compatible with R10.4.1 flow cells (FLO-PRO114M).
After taking the flow cell out of the fridge, wait 20 minutes for the flow cell to reach room temperature before inserting it into the PromethION. Condensation can form on the flow cell in humid environments. Inspect the gold connector pins on the top and underside of the flow cell for condensation and wipe off with a lint-free wipe if any is observed. Ensure the heat pad (black pad) is present on the underside of the flow cell.
Thaw the Sequencing Buffer (SB), Library Beads (LIB), Flow Cell Tether (FCT) and Flow Cell Flush (FCF) at room temperature before mixing by vortexing. Then spin down and store on ice.
To prepare the flow cell priming mix, combine Flow Cell Tether (FCT) and Flow Cell Flush (FCF), as directed below. Mix by vortexing at room temperature.
In a clean suitable tube for the number of flow cells, combine the following reagents:
| Reagent | Volume per flow cell |
|---|---|
| Flow Cell Flush (FCF) | 1,170 µl |
| Flow Cell Tether (FCT) | 30 µl |
| Total volume | 1,200 µl |
For PromethION 2 Solo, load the flow cell(s) as follows:
Place the flow cell flat on the metal plate.
Slide the flow cell into the docking port until the gold pins or green board cannot be seen.

For the PromethION 24/48, load the flow cell(s) into the docking ports:
- Line up the flow cell with the connector horizontally and vertically before smoothly inserting into position.
- Press down firmly onto the flow cell and ensure the latch engages and clicks into place.


Insertion of the flow cells at the wrong angle can cause damage to the pins on the PromethION and affect your sequencing results. If you find the pins on a PromethION position are damaged, please contact support@nanoporetech.com for assistance.

Complete a flow cell check to assess the number of pores available before loading the library.
This step can be omitted if the flow cell has been checked previously.
See the flow cell check document for more information.
Slide the inlet port cover clockwise to open.

Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the inlet port, draw back a small volume to remove any air bubbles:
- Set a P1000 pipette tip to 200 µl.
- Insert the tip into the inlet port.
- Turn the wheel until the dial shows 220-230 µl, or until you see a small volume of buffer entering the pipette tip.

Load 500 µl of the priming mix into the flow cell via the inlet port, avoiding the introduction of air bubbles. Wait five minutes. During this time, prepare the library for loading using the next steps in the protocol.

Thoroughly mix the contents of the Library Beads (LIB) by pipetting.
The Library Beads (LIB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
We recommend using the Library Beads (LIB) for most sequencing experiments. However, the Library Solution (LIS) is available for more viscous libraries.
In a new 1.5 ml Eppendorf DNA LoBind tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SB) | 100 µl |
| Library Beads (LIB) thoroughly mixed before use | 68 µl |
| DNA library | 32 µl |
| Total | 200 µl |
Note: The prepared library is used for loading into the flow cell. Store the library on ice or at 4°C until ready to load.
Complete the flow cell priming by slowly loading 500 µl of the priming mix into the inlet port.

Mix the prepared library gently by pipetting up and down just prior to loading.
Load 200 µl of library into the inlet port using a P1000 pipette.

Close the valve to seal the inlet port.
For optimal sequencing output, install the light shield on your flow cell as soon as the library has been loaded.
We recommend leaving the light shield on the flow cell when library is loaded, including during any washing and reloading steps. The shield can be removed when the library has been removed from the flow cell.
If the light shield has been removed from the flow cell, install the light shield as follows:
- Align the inlet port cut out of the light shield with the inlet port cover on the flow cell. The leading edge of the light shield should sit above the flow cell ID.
- Firmly press the light shield around the inlet port cover. The inlet port clip will click into place underneath the inlet port cover.


Close the PromethION lid when ready to start a sequencing run on MinKNOW.
Wait a minimum of 10 minutes after loading the flow cells onto the PromethION before initiating any experiments. This will help to increase the sequencing output.
For instructions on setting up your sequencing run please visit the data acquisition and basecalling section of this protocol.
Reminder: For this protocol, we recommend washing and reloading your flow cell with fresh library to maintain high data acquisition after ~22 hours of sequencing.
Follow the instructions in the washing and reloading a PromethION Flow Cell section of this protocol.
9. Washing and reloading a PromethION Flow Cell
材料
- 之前步骤获得的已连接接头的 DNA 文库
- 测序芯片清洗剂盒(EXP-WSH004)
- 测序辅助扩展包 V14(EXP-AUX003)
耗材
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- P1000 移液枪和枪头
- P20 移液枪和枪头
- 盛有冰的冰桶
- 涡旋混匀仪
We recommend washing and reloading the flow cell after ~22 hours of sequencing.
For this method, the flow cell is washed after ~22 hours of sequencing to restore pores to ensure efficient data acquisition. After an additional 22 hours of sequencing, the flow cell is washed and reloaded a second time. For this reason, enough library was generated for 3 flow cell loads in the adapter ligation step of the protocol.
- This washing procedure aims to remove most of the initial library and unblock the pores to prepare the flow cell for the loading of a subsequent library.
- Data acquisition in MinKNOW should be paused during the wash procedure and library loading.
- After the flow cell has been washed, the next library can be loaded.
You can navigate to the Pore Activity or the Pore Scan Results plot to see pore availability.
Below you can find example data for pore states observed on a flow cell before and after wash steps are performed. Additionally, you can observe an example for the cummulative sequencing data output, including the wash and reload steps. The red asterisks indicate the flow cell wash and reloads.

Figure 1. Channel state over a 100 hour run. The flow cell washes are incorporated into the method to restore blocked pores, to allow continuous data acquisition. Red asterisks denote when a flush was performed.

Figure 2. Cumulative sequence data output, over a 100 hour run. Red asterisks denote when a flush was performed.

Figure 3. Read length profile for a 30 kb N50 library. The approximately gaussian shape is characteristic of gDNA that has undergone SFE size selection and Megaruptor shearing. The short distribution of reads at ~5kb is due to premature termination of reads at the mux scan.
Washing and reloading a PromethION Flow Cell video
This video will show you how to wash a flow cell after a sequencing run and how to load a new library.
We recommend keeping the light shield on the flow cell during washing if a second library will be loaded straight away.
If the flow cell is to be washed and stored, the light shield can be removed.
Place the tube of Wash Mix (WMX) on ice. Do not vortex the tube.
Thaw one tube of Wash Diluent (DIL) at room temperature.
Mix the contents of Wash Diluent (DIL) thoroughly by vortexing, then spin down briefly and place on ice.
In a fresh 1.5 ml Eppendorf DNA LoBind tube, prepare the following Flow Cell Wash Mix:
| Reagent | Volume per flow cell |
|---|---|
| Wash Mix (WMX) | 2 μl |
| Wash Diluent (DIL) | 398 μl |
| Total | 400 μl |
Mix well by pipetting, and place on ice. Do not vortex the tube.
Pause the sequencing experiment in MinKNOW, and leave the flow cell in the device.
It is vital that the inlet port is closed before removing waste to prevent air from being drawn across the sensor array area, which would lead to a significant loss of sequencing channels.
Remove waste buffer, as follows:
- Close the inlet port.
- Insert a P1000 pipette into a waste port and remove the waste buffer.
Note: As both the inlet port is closed, no fluid should leave the sensor array area.
Slide the inlet port cover clockwise to open the inlet port.

Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the inlet port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the inlet port
- Turn the wheel until the dial shows 220-230 µl, or until you can see a small volume of buffer entering the pipette tip.

Slowly load 200 µl of the prepared flow cell wash mix into the inlet port, as follows:
- Using a P1000 pipette, take 200 µl of the flow cell wash mix
- Insert the pipette tip into the inlet port, ensuring there are no bubbles in the tip
- Slowly twist the pipette wheel down to load the flow cell (if possible with your pipette) or push down the plunger very slowly, leaving a small volume of buffer in the pipette tip.
- Set a timer for a 5 minute incubation.
Once the 5 minute incubation time is complete, carefully load the remaining 200 µl of the prepared flow cell wash mix into the inlet port, as follows:
- Using a P1000 pipette, take 200 µl of the flow cell wash mix
- Insert the pipette tip into the inlet port, ensuring there are no bubbles in the tip
- Slowly twist the pipette wheel down to load the flow cell (if possible with your pipette) or push down the plunger very slowly, leaving a small volume of buffer in the pipette tip.
Close the inlet port and wait for 1 hour.
It is vital that the inlet port is closed before removing waste to prevent air from being drawn across the sensor array area, which would lead to a significant loss of sequencing channels.
Remove the waste buffer, as follows:
- Ensure the inlet port is closed.
- Insert a P1000 pipette into a waste port and remove the waste buffer
Note: As the inlet port is closed, no fluid should leave the sensor array area.
The buffers used in this process are incompatible with conducting a Flow Cell Check step prior to loading the subsequent library. However, number of available pores will be reported after the next pore scan.
Thaw the Sequencing Buffer (SB), Library Beads (LIB) or Library Solution (LIS, if using), Flow Cell Tether (FCT) and Flow Cell Flush (FCF) at room temperature, before mixing by vortexing. Then spin down before storing on ice.
Prepare the flow cell priming mix in a suitable tube for the number of flow cells to flush. Once combined, mix well by briefly vortexing.
| Reagents | Volume per flow cell |
|---|---|
| Flow Cell Flush (FCF) | 1,170 µl |
| Flow Cell Tether (FCT) | 30 µl |
| Total volume | 1,200 µl |
Slide the inlet port cover clockwise to open.

Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the inlet port, draw back a small volume to remove any air bubbles:
- Set a P1000 pipette tip to 200 µl.
- Insert the tip into the inlet port.
- Turn the wheel until the dial shows 220-230 µl, or until you see a small volume of buffer entering the pipette tip.

Slowly load 500 µl of the priming mix into the inlet port, as follows:
- Using a P1000 pipette, take 500 µl of the priming mix
- Insert the pipette tip into the priming port, ensuring there are no bubbles in the tip
- Slowly twist the pipette wheel down to load the flow cell (if possible with your pipette) or push down the plunger very slowly, leaving a small volume of buffer in the pipette tip.

It is vital to wait five minutes between the priming mix flushes to ensure effective removal of the nuclease.
Close the inlet port and wait five minutes.
During this time, prepare the library for loading using the next steps in the protocol.
Thoroughly mix the contents of the Library Beads (LIB) by pipetting.
The Library Beads (LIB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
We recommend using the Library Beads (LIB) for most sequencing experiments. However, the Library Solution (LIS) is available for more viscous libraries.
In a new 1.5 ml Eppendorf DNA LoBind tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SB) | 100 µl |
| Library Beads (LIB) thoroughly mixed before use, or Library Solution (LIS) | 68 µl |
| DNA library | 32 µl |
| Total | 200 µl |
Note: Library loading volume has been increased to improve array coverage.
It is vital that the inlet port is closed before removing waste to prevent air from being drawn across the sensor array area, which would lead to a significant loss of sequencing channels.
Remove the waste buffer, as follows:
- Ensure the inlet port is closed.
- Insert a P1000 pipette into a waste port and remove the waste buffer
Note: As the inlet port is closed, no fluid should leave the sensor array area.
Slide the inlet port cover clockwise to open.

Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the inlet port, draw back a small volume to remove any air bubbles:
- Set a P1000 pipette tip to 200 µl.
- Insert the tip into the inlet port.
- Turn the wheel until the dial shows 220-230 µl, or until you see a small volume of buffer entering the pipette tip.

Slowly load 500 µl of the priming mix into the inlet port, as follows:
- Using a P1000 pipette, take 500 µl of the priming mix
- Insert the pipette tip into the priming port, ensuring there are no bubbles in the tip
- Slowly twist the pipette wheel down to load the flow cell (if possible with your pipette) or push down the plunger very slowly, leaving a small volume of buffer in the pipette tip.

It is vital that the inlet port is closed before removing waste to prevent air from being drawn across the sensor array area, which would lead to a significant loss of sequencing channels.
Remove waste buffer, as follows:
- Close the inlet port.
- Insert a P1000 pipette into a waste port and remove the waste buffer.
Note: As both the inlet port is closed, no fluid should leave the sensor array area.
Slide the inlet port cover clockwise to open.

Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
After opening the inlet port, draw back a small volume to remove any air bubbles:
- Set a P1000 pipette tip to 200 µl.
- Insert the tip into the inlet port.
- Turn the wheel until the dial shows 220-230 µl, or until you see a small volume of buffer entering the pipette tip.

Mix the prepared library gently by pipetting up and down just prior to loading.
Load 200 µl of library into the inlet port using a P1000 pipette.

Close the valve to seal the inlet port.
For optimal sequencing output, install the light shield on your flow cell as soon as the library has been loaded.
We recommend leaving the light shield on the flow cell when library is loaded, including during any washing and reloading steps. The shield can be removed when the library has been removed from the flow cell.
If the light shield has been removed from the flow cell, install the light shield as follows:
- Align the inlet port cut out of the light shield with the inlet port cover on the flow cell. The leading edge of the light shield should sit above the flow cell ID.
- Firmly press the light shield around the inlet port cover. The inlet port clip will click into place underneath the inlet port cover.


Close the PromethION lid when ready to start a sequencing run on MinKNOW.
Wait a minimum of 10 minutes after loading the flow cells onto the PromethION before initiating any experiments. This will help to increase the sequencing output.
Perform the "Washing and reloading a PromethION flow cell" step twice for a total of three library loads (initial library load + two wash and reloads) to maximise data acquisition.
- The first wash and reload should be performed at ~22 hours into sequencing.
- The second wash and reload should be performed at ~44 hours into sequencing.
10. Data acquisition and basecalling
How to start sequencing
The sequencing device control, data acquisition and real-time basecalling are carried out by the MinKNOW software.
We recommend basecalling with the high accuracy (HAC) basecaller in real-time with BAM selected as output type using the P2 Solo or P24/P48 device.
You must generate a BAM file from your sequening run, as this is required for input into the wf-human-variation workflow.
Refer to the links below containing the detailed instructions for setting up the device and sequencing run:
PromethION 24 and 48: "Starting a sequencing run with PromethION 24 and 48"
PromethION 2 Solo: "Starting a sequencing run on PromethION 2 Solo"
Below are the recommended sequencing parameters for MinKNOW.
MinKNOW settings for 30kb human sample on PromethION
For inputs of 30 kb, we recommend increasing the run time to 100 hours to accommodate for the two flow cell washes, using the modified bases option for basecalling and ensuring a BAM output is selected. All other sequencing parameters can be kept to their default settings. Below are our current recommendations:
Positions
Flow cell position: [user defined]
Experiment name: [user defined]
Flow cell type: FLO-PRO114M
Sample ID: [user defined]
Kit
Kit selection: Ligation Sequencing Kit (SQK-LSK114)
Run configuration
Sequencing and analysis
Basecalling: On [default] Modified bases: On with '5mC & 5hmC CG contexts' selected Model: High-accuracy basecalling (HAC) [default]
Barcoding: Disabled [default]
Alignment: Off [default]
We do not currently recommend live alignment during sequencing, as it can slow down system processing.
Adaptive sampling: Off [default]
Advanced options Active channel selection: On [default] Time between pore scans: 1.5 [default] Reserve pores: On [default]
Data targets
Run limit: 100 hours [default]
Output
Output format .POD5: On [default] .FASTQ: On [default] .BAM: On
Filtering: On [default] Qscore: 9 [default] Minimum read length: 200 bp [default]
We do not recommend live alignment during sequencing, as it can slow down system processing.
You can align your BAM file post-sequencing by following one of the methods below:
| Aligning the BAM file in MinKNOW | Aligning the BAM file during the wf-human-variation workflow |
|---|---|
| Align the BAM output after live basecalling in MinKNOW. This will prevent slowing down your sytems processing. The aligned BAM file can be used as your file input in the wf-human-variation workflow. Using mapped BAM as input, the workflow will take 1-2 hours. | You can provide a reference genome along with the unaligned BAM file during the wf-human-variation workflow set-up. Using an unmapped BAM is used as input, the workflow will take approximately 5-8 hours. |
Further information is available in the Downstream analysis section of this protocol.
11. Downstream analysis
Analysis of human cell DNA sequence data
For the analysis of human cell DNA sequence data, we recommend the wf-human-variation workflow. This end-to-end software pipeline is implemented using the Nextflow workflow language and implements methods for the calling of single nucleotide polymorphisms (SNPs), structural variants (SVs), and for reporting DNA methylation information.
The wf-human-variation workflow is best run from the BAM file produced by MinKNOW when the modified base model for basecalling is selected. If sequence read mapping to the reference genome is not performed by MinKNOW, we recommend to perform the basecalling using the wf-basecalling workflow. Ensure you save the outputs in BAM format by providing the --output_bam option.
The tools below are used in the analysis workflow and can be run in isolation or together:
Sniffles2 calls SVs and file output include an HTML report of QC metrics and VCF format list of variants and their quality scores.
Clair3 calls SNPs and file output includes an HTML report of QC metrics and VCF format list of variants and their quality scores.
modkit extracts methylation information from the provided BAM file which is summarised in a BEDmethyl format file.
The wf-human-variation workflow is preconfigured using appropriate parameters and requires tuning only for the choice of reference genome and Clair3 model. Please see the project’s documentation for further details.
The results from the wf-human-variation workflow can be further explored by viewing in a track-based genome browser such as IGV can be assessed for known pathogenicity through tertiary analysis software.
EPI2ME analysis workflow
The wf-human-variation workflow is intended to be run using the Nextflow software.
We recommend performing downstream analysis using EPI2ME which facilitates bioinformatic analyses by allowing users to run Nextflow workflows in a Graphical User Interfaces (GUI). EPI2ME maintains a collection of bioinformatic workflows which are curated and actively maintained by experts in long-read sequence analysis. The collection of all our available EPI2ME workflows can be found here.
For new users, the quick start guide can be found here outlining how to use this interface.
Compute requirements for the wf-human-variation workflow on EPI2ME
| Recommended requirements | Minimum requirements |
|---|---|
| CPUs = 32 | CPUs = 16 |
| Memory = 128GB | Memory = 32GB |
Approximate run time: Variable depending on whether it is targeted sequencing or whole genome sequencing, as well as coverage and the individual analyses requested. For instance, a 90X human sample run (options: --snp --sv --mod --str --cnv --phased --sex male) takes less than 8h with recommended resources.
ARM processor support: False
The wf-human-variation workflow can also be run using the command line interface (CLI)
Please see the Github page for further details.
Note: We only recommend the command line interface (CLI) for experienced users.
Open the EPI2ME app using the desktop shortcut.
On the landing page, open the workflow tab on the left-hand sidebar.

Navigate to the Available workflows tab and click on wf-human-variation option.

Click install.

Navigate to the Installed tab and click on the installed wf-human-variation workflow.
If the workflow was already installed, check for updates by clicking 'Update workflow'.
We recommend running the latest version of our workflows for the best results.

Click on Run this workflow to open the launch wizard.
Select the environment you are running the workflow from:

Click on the sub-workflow(s) you want to run in the wf-human-variation analysis.

You must have at least one of the sub-workflows selected to proceed with analysis.

Note: For more information on the sub-workflows click on the "Expand" option in the platform, or visit our online EPI2ME documentation.
Navigate to the 'Main options' tab to assign a 'Sample name' as an identifier in workflow outputs.

The wf-human-variation workflow uses sequencing data in the form of a single BAM file or a folder of BAM files.
The BAM files used as an input can be aligned or unaligned:
| Aligning the BAM file in MinKNOW (prior to the wf-human-variation workflow) | Aligning the BAM file during the wf-human-variation workflow (during the wf-human-variation workflow) |
|---|---|
| Align the BAM output after live basecalling in MinKNOW. This will prevent slowing down your sytems processing. The aligned BAM file can be used as your file input in the wf-human-variation workflow. For more information on post-run alignment in MinKNOW please visit our MinKNOW protocol. Using mapped BAM as input, the workflow will take 1-2 hours. | You can provide a reference genome along with the unaligned BAM file during the wf-human-variation workflow set-up. Using an unmapped BAM is used as input, the workflow will take approximately 5-8 hours. |
In the 'Main options' upload your sequencing data in the form of a single BAM file or a folder of BAM files.

If you have an unaligned BAM file as input, in the 'Main options' upload your reference genome in FASTA format.

Click Launch workflow.
Ensure all parameter options have green ticks.

Once the wf-human-variation workflow finishes, a report will be produced alongside output files.
wf-human-variation workflow outputs
The primary workflow outputs include:
- gzipped VCF file containing the SNPs in the dataset from
--snp - gzipped VCF file containing the SVs in the dataset from
--sv - gzipped bedMethyl file aggregating modified base counts from
--mod - HTML report detailing the primary findings of the workflow for QC metrics, and SNP and SV calling
- If an unaligned BAM file was provided, the workflow will ouput a CRAM file containing the alignments used to make the downstream variant calls.
The secondary workflow outputs:
mosdepthouputs include:{sample_name}.mosdepth.global.dist.txt: a cumulative distribution indicating the proportion of total bases for each and all reference sequences{sample_name}.regions.bed.gz: the mean coverage for each region in the provided BED file{sample_name}.thresholds.bed.gz: the number of bases in each region that are covered at or above each threshold value (1, 10, 20, 30X)
- bamstats ouputs include:
{sample_name}.readstats.tsv.gz: a gzipped TSV summarising per-alignment statistics produced by bamstats{sample_name}.ftagstat.tsv: a text file with summary alignment statistics for each reference sequence
wf-human-variation workflow tips
It is possible to phase SNPs, SVs and modified bases by providing the --phased option.
To improve the accuracy of SV calling, specify a suitable tandem repeat BED for your reference with --tr_bed.
Aggregation of methylation calls with --mod requires data to be basecalled with a model that includes base modifications, providing the MM and ML BAM tags. To do so on MinKNOW, ensure 'Modified bases' option is selected during basecalling set up, with the '5mC' model selected.
Ensure to retain the input reference when basecalling or alignment is performed as CRAM files cannot be read without the corresponding input reference.
For a full list of available basecalling models, refer to the Dorado documentation.
12. Flow cell reuse and returns
We do not recommend washing and reusing your flow cells for another experiment, if using this protocol.
Due to the extended sequencing time, and the multiple flow cell washes and library reloads, we do not recommend reusing the flow cells.
Reusing these flow cells for subsequent sequencing experiments may result in insufficient data generation for analysis.
Follow the returns procedure to send back the flow cells to Oxford Nanopore for recycling.
Instructions for returning flow cells can be found here.
If you encounter issues or have questions about your sequencing experiment, please refer to the Troubleshooting guide in this protocol.
13. Issues during DNA extraction and library preparation
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Low sample quality
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low DNA purity (Nanodrop reading for DNA OD 260/280 is <1.8 and OD 260/230 is <2.0–2.2) | The DNA extraction method does not provide the required purity | The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. Consider performing an additional AMPure bead clean-up step. |
Low DNA recovery after AMPure bead clean-up
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Low recovery | DNA loss due to a lower than intended AMPure beads-to-sample ratio | 1. AMPure beads settle quickly, so ensure they are well resuspended before adding them to the sample. 2. When the AMPure beads-to-sample ratio is lower than 0.4:1, DNA fragments of any size will be lost during the clean-up. |
| Low recovery | DNA fragments are shorter than expected | The lower the AMPure beads-to-sample ratio, the more stringent the selection against short fragments. Please always determine the input DNA length on an agarose gel (or other gel electrophoresis methods) and then calculate the appropriate amount of AMPure beads to use. 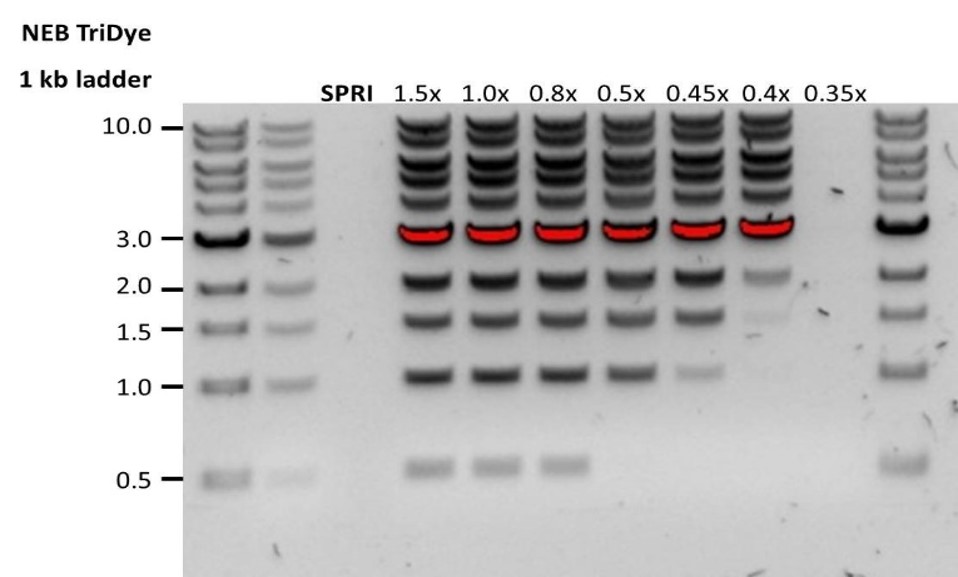 |
| Low recovery after end-prep | The wash step used ethanol <70% | DNA will be eluted from the beads when using ethanol <70%. Make sure to use the correct percentage. |
14. Issues during the sequencing run
Below is a list of the most commonly encountered issues, with some suggested causes and solutions.
We also have an FAQ section available on the Nanopore Community Support section.
If you have tried our suggested solutions and the issue still persists, please contact Technical Support via email (support@nanoporetech.com) or via LiveChat in the Nanopore Community.
Fewer pores at the start of sequencing than after Flow Cell Check
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | An air bubble was introduced into the nanopore array | After the Flow Cell Check it is essential to remove any air bubbles near the priming port before priming the flow cell. If not removed, the air bubble can travel to the nanopore array and irreversibly damage the nanopores that have been exposed to air. The best practice to prevent this from happening is demonstrated in this video for how to load a PromethION Flow Cell. |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | The flow cell is not correctly inserted into the device | Stop the sequencing run, remove the flow cell from the sequencing device and insert it again, checking that the flow cell is firmly seated in the device and that it has reached the target temperature. If applicable, try a different position on the device (GridION/PromethION). |
| MinKNOW reported a lower number of pores at the start of sequencing than the number reported by the Flow Cell Check | Contaminations in the library damaged or blocked the pores | The pore count during the Flow Cell Check is performed using the QC DNA molecules present in the flow cell storage buffer. At the start of sequencing, the library itself is used to estimate the number of active pores. Because of this, variability of about 10% in the number of pores is expected. A significantly lower pore count reported at the start of sequencing can be due to contaminants in the library that have damaged the membranes or blocked the pores. Alternative DNA/RNA extraction or purification methods may be needed to improve the purity of the input material. The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
MinKNOW script failed
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Script failed" | Restart the computer and then restart MinKNOW. If the issue persists, please collect the MinKNOW log files and contact Technical Support. If you do not have another sequencing device available, we recommend storing the flow cell and the loaded library at 4°C and contact Technical Support for further storage guidance. |
Pore occupancy below 40%
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Pore occupancy <40% | Not enough library was loaded on the flow cell | For the human genome sequencing protocols, 200-300 ng of good quality library should be loaded on to an R10.4.1 flow cell to keep pore occupancy high. |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and sequencing adapters did not ligate to the DNA | Make sure to use the NEBNext Quick Ligation Module (E6056) and Oxford Nanopore Technologies Ligation Buffer (LNB, provided in the SQK-LSK114 kit) at the sequencing adapter ligation step, and use the correct amount of each reagent. A Lambda control library can be prepared to test the integrity of the third-party reagents. |
| Pore occupancy close to 0 | The Ligation Sequencing Kit was used, and ethanol was used instead of LFB or SFB at the wash step after sequencing adapter ligation | Ethanol can denature the motor protein on the sequencing adapters. Make sure the LFB or SFB buffer was used after ligation of sequencing adapters. |
| Pore occupancy close to 0 | No tether on the flow cell | Tethers are adding during flow cell priming (FCT tube). Make sure FCT was added to FCF before priming. |
Shorter than expected read length
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Shorter than expected read length | Unwanted fragmentation of DNA sample | Read length reflects input DNA fragment length. Input DNA can be fragmented during extraction and library prep. 1. Please review the Extraction Methods in the Nanopore Community for best practice for extraction. 2. Visualise the input DNA fragment length distribution on an agarose gel before proceeding to the library prep. 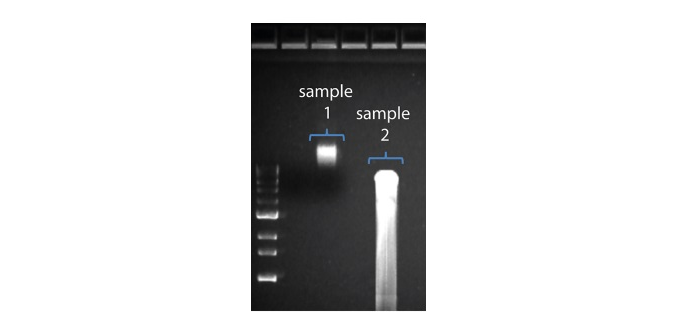 In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented. In the image above, Sample 1 is of high molecular weight, whereas Sample 2 has been fragmented.3. During library prep, avoid pipetting and vortexing when mixing reagents. Flicking or inverting the tube is sufficient. |
Large proportion of unavailable pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
Large proportion of unavailable pores (shown as blue in the channels panel and pore activity plot) 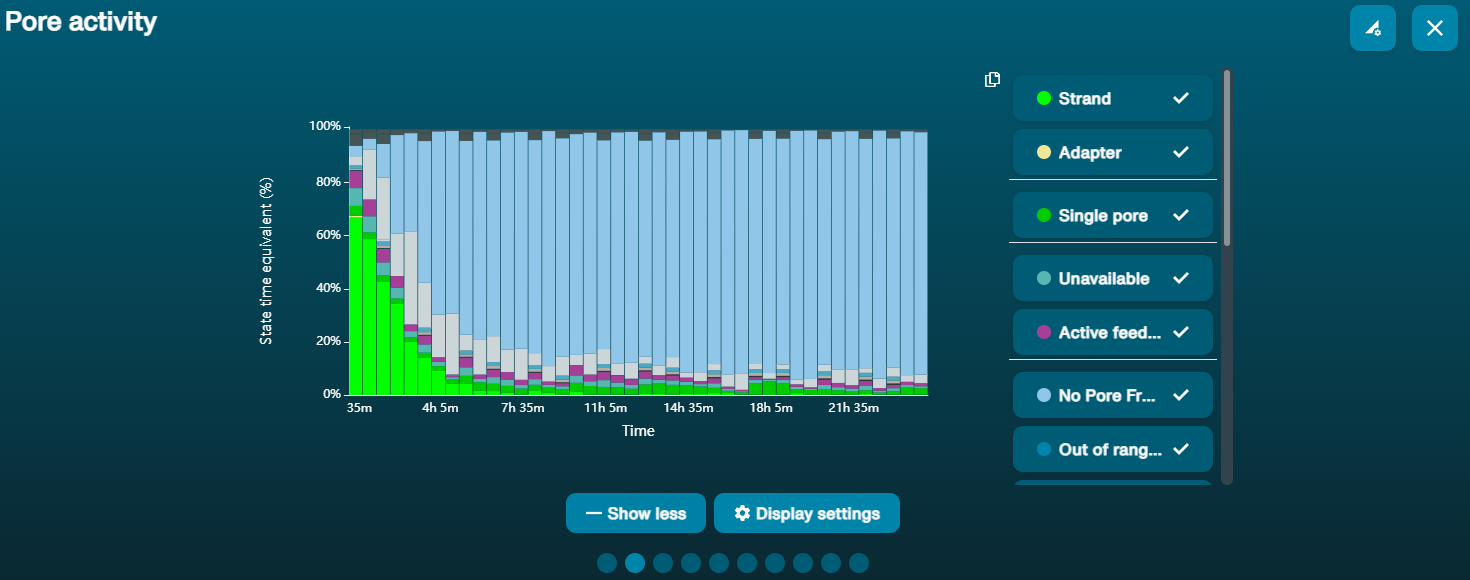 The pore activity plot above shows an increasing proportion of "unavailable" pores over time. The pore activity plot above shows an increasing proportion of "unavailable" pores over time. | Contaminants are present in the sample | Some contaminants can be cleared from the pores by the unblocking function built into MinKNOW. If this is successful, the pore status will change to "sequencing pore". If the portion of unavailable pores stays large or increases: 1. A nuclease flush using the Flow Cell Wash Kit (EXP-WSH004) can be performed, or 2. Run several cycles of PCR to try and dilute any contaminants that may be causing problems. |
Large proportion of inactive pores
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Large proportion of inactive/unavailable pores (shown as light blue in the channels panel and pore activity plot. Pores or membranes are irreversibly damaged) | Air bubbles have been introduced into the flow cell | Air bubbles introduced through flow cell priming and library loading can irreversibly damage the pores. Watch the how to load a PromethION Flow Cell video for best practice. |
| Large proportion of inactive/unavailable pores | Certain compounds co-purified with DNA | Known compounds, include polysaccharides. 1. Clean-up using the QIAGEN PowerClean Pro kit. 2. Perform a whole genome amplification with the original gDNA sample using the QIAGEN REPLI-g kit. |
| Large proportion of inactive/unavailable pores | Contaminants are present in the sample | The effects of contaminants are shown in the Contaminants Know-how piece. Please try an alternative extraction method that does not result in contaminant carryover. |
Temperature fluctuation
| Observation | Possible cause | Comments and actions |
|---|---|---|
| Temperature fluctuation | The flow cell has lost contact with the device | Check that there is a heat pad covering the metal plate on the back of the flow cell. Re-insert the flow cell and press it down to make sure the connector pins are firmly in contact with the device. If the problem persists, please contact Technical Services. |
Failed to reach target temperature
| Observation | Possible cause | Comments and actions |
|---|---|---|
| MinKNOW shows "Failed to reach target temperature" | The instrument was placed in a location that is colder than normal room temperature, or a location with poor ventilation (which leads to the flow cells overheating) | MinKNOW has a default timeframe for the flow cell to reach the target temperature. Once the timeframe is exceeded, an error message will appear and the sequencing experiment will continue. However, sequencing at an incorrect temperature may lead to a decrease in throughput and lower q-scores. Please adjust the location of the sequencing device to ensure that it is placed at room temperature with good ventilation, then re-start the process in MinKNOW. |







