使用 SQK-LSK114 对人类细胞系来源的 10 kb 样本进行基因组变异测序 (GDH_9173_v114_revR_10Sep2025)
PromethION: Protocol
使用 SQK-LSK114 对人类细胞系来源的 10 kb 样本进行基因组变异测序 V GDH_9173_v114_revR_10Sep2025
本实验指南具有以下特点:
- 从人类细胞系中提取、N50 约为 10 kb 的基因组 DNA
- 样本制备时间约 220 分钟,文库制备时间约 90 分钟
- 数据分析时间约 1–2 小时
- 无需 PCR 扩增
- 兼容 R10.4.1 测序芯片
仅供研究使用
FOR RESEARCH USE ONLY
概览
本实验指南具有以下特点:
- 从人类细胞系中提取、N50 约为 10 kb 的基因组 DNA
- 样本制备时间约 220 分钟,文库制备时间约 90 分钟
- 数据分析时间约 1–2 小时
- 无需 PCR 扩增
- 兼容 R10.4.1 测序芯片
仅供研究使用
1. 实验方案概览
使用 SQK-LSK114 对人类细胞系来源的 10 kb 样本进行基因组变异测序 实验指南简介
本方案描述了从培养细胞中提取基因组 DNA(gDNA)、进行文库构建和测序,并使用 EPI2ME 中的 “Human variant workflow”(人类基因组变异工作流程) 对数据进行分析的完整流程。该流程可用于检测结构变异(SVs)和单核苷酸变异(SNVs),这些变异对于理解遗传多样性、疾病机制和进化过程具有重要意义。本方案旨在制备读长 N50 约为 10 kb 的测序文库,并获得约 30–40× 的全基因组覆盖度,以实现对小变异和大变异的可靠检测,同时支持 DNA 甲基化分析和单倍型分型。
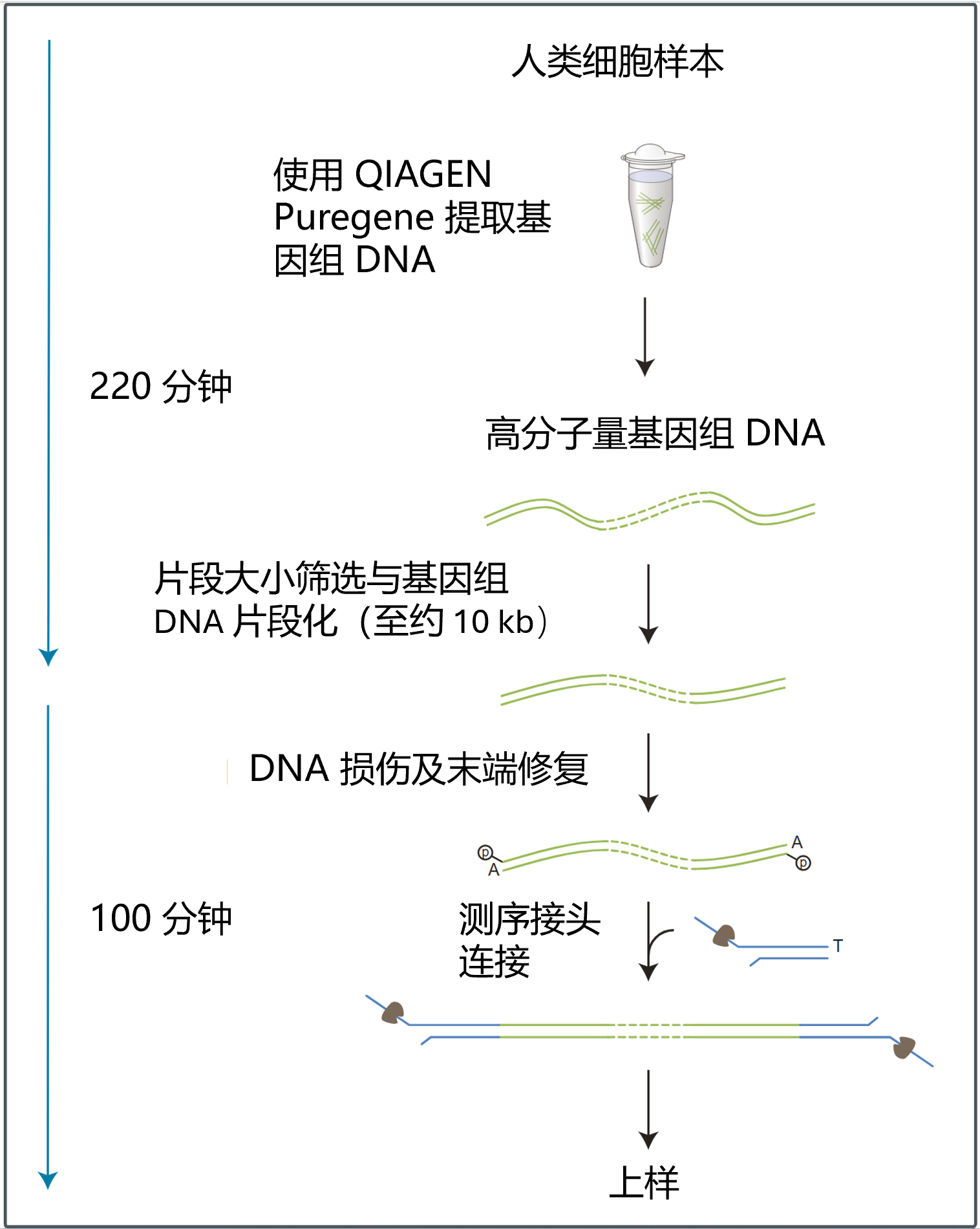
简要流程如下:使用 QIAGEN Puregene Cell Kit 从 500 万个培养细胞中提取基因组 DNA。 随后,使用 Covaris g-TUBE™ 对 DNA 进行机械打断。打断后的 DNA 采用 连接测序试剂盒 V14(SQK-LSK114) 进行文库构建,并最终在 PromethION 平台上完成测序。
测序过程中,原始数据经 MinKNOW 完成碱基识别和序列比对,生成比对后的 BAM 文件。随后,使用 wf-human-variation 工作流程对 BAM 数据进行分析。该流程结合 Sniffles2、Clair3 及 modbam2bed 等工具,用于检测结构变异(SVs)和单核苷酸多态性(SNPs),并输出 DNA 甲基化分析结果。
测序工作流程:
实验准备
您将需要:
- 提取起始样本(细胞)。
- 确保您已准备好测序试剂盒、正确的仪器以及第三方试剂。
- 下载数据收集和分析软件。
- 检查您的测序芯片上有足够的活性纳米孔,以确保测序良好运行。
样本制备
照文中所述的提取方法从细胞中提取 gDNA,并使用 Covaris g-TUBE 对所得 gDNA 进行打断。
评估其长度、浓度和纯度。 质量评估步骤对确保实验成功至关重要。
文库制备
下表概述了文库制备所需的步骤,包括时间安排和可以中止的节点。
| 实验步骤 | 流程 | 时间 | 中止节点 |
|---|---|---|---|
| DNA 损伤及末端修复 | 修复 DNA,并对 DNA 进行末端修复以便与接头连接 | 35 分钟 | 4°C 过夜 |
| 接头连接及纯化 | 将测序接头连接到 DNA 末端 | 55 分钟 | 若为短期保存或重复使用(例如在清洗芯片后再次上样),建议4℃保存; 若为长期储存,建议-80℃保存。 我们强烈建议在连接测序接头后即对文库进行测序。 |
| 测序芯片的预处理及上样 | 对测序芯片进行预处理,然后将制备好的文库加至芯片中进行测序 | 10 分钟 |
测序和分析
您将需要:
- 使用 MinKNOW 软件开始测序。该软件会通过测序仪收集原始数据,并将其识别成碱基序列。
- 通过 wf-human-variation 工作流程进行数据分析。
实验方案适用性
本实验方案只适用于与以下产品搭配使用:
- 连接测序试剂盒 V14(SQK-LSK114)
- R10.4.1 PromethION 测序芯片(FLO-PRO114M)
- 短片段去除扩展包(EXP-SFE001)
- 测序芯片清洗试剂盒(EXP-WSH004)
- 测序辅助扩展包 V14(EXP-AUX003)
- PromethION 24/48 测序设备 - PromethION IT 配置要求文档
- PromethION 2 Solo 测序设备 - PromethION 2 Solo IT 配置要求文档
2. 仪器及耗材
材料
- (用于提取)5 × 10⁶ 个细胞(例如细胞培养物或组织样本)
- (用于文库制备)1 µg 经片段化处理的基因组 DNA
- 连接测序试剂盒V14(SQK-LSK114)
耗材
- PromethION 测序芯片
- 供 Oxford Nanopore Technologies® 连接测序使用的 NEBNext® 配套模块 v2(NEB, E7672S 或 E7672L)
- Puregene 细胞试剂盒(QIAGEN,158043)
- g-TUBE™(Covaris,520079)
- 15 ml Falcon离心管
- 2 ml Eppendorf DNA LoBind 离心管
- 1.5 ml Eppendorf DNA LoBind 离心管
- 0.2 ml 薄壁PCR管
- 无核酸酶水(如ThermoFisher,AM9937)
- 新制备的 80% 乙醇(用无核酸酶水配制)
- 异丙醇,100%(Fisher, 10723124)
- 磷酸盐缓冲盐溶液(PBS),pH 7.4(ThermoFisher,10010023)
- TE 缓冲液(10 mM Tris-HCl、1 mM EDTA、pH 8.0)(Fisher scientific,10224683)
- (非必需)TE 缓冲液(10 mM Tris-HCl、0.1 mM EDTA,pH 8.0)
- 接种环或一次性镊子
- Qubit™ 分析管(Invitrogen, Q32856)
- Qubit dsDNA BR Assay(双链DNA宽范围检测)试剂盒(ThermoFisher ,Q32850)
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
- Agilent 基因组 DNA 165 kb 分析试剂盒(Agilent,FP-1002-0275)
仪器
- PromethION 测序设备
- PromethION 测序芯片遮光片
- Hula混匀仪(低速旋转式混匀仪)
- 适用于1.5ml Eppendorf 离心管的磁力架
- 设定为 37°C 和 50°C 的培养箱或水浴锅
- 适用于 15 ml Falcon 管的离心机及转子
- 迷你离心机
- 涡旋混匀仪
- 热循环仪
- 宽口移液枪头
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- P2 移液枪和枪头
- 盛有冰的冰桶
- 计时器
- Agilent Femto Pulse 系统(或用于读长质控的等效仪器)
- Qubit™ 荧光计(或用于质控检测的等效仪器)
上述材料、耗材及仪器清单适用于本指南中样本制备阶段的 DNA 提取流程及后续文库构建流程。对于已完成 DNA 提取的样本,仅需准备文库构建阶段所需的材料。
文库构建需使用 3 μg 基因组 DNA,且 N50 为 10 kb。
本端到端工作流程建议在样本制备阶段采用 QIAGEN Puregene 细胞试剂盒,从 5 × 10⁶ 个细胞(如细胞培养物或组织样本)中提取高分子量人类基因组 DNA(gDNA)。
此外,您也可按需采用其他提取方案,但需注意,Oxford Nanopore Technologies 未对其进行验证。
起始 DNA
DNA 质控
选择符合质量和浓度要求的起始 DNA 至关重要。使用过少或过多的 DNA,或者质量较差的 DNA(如,高度碎片化、含有 RNA 或化学污染物的 DNA)都会影响文库制备。
有关如何对 DNA 样品进行质控,请参考起始 DNA/RNA 质控实验指南。
化学污染物
从原始样本中提取 DNA 的方法不同,可能会导致经纯化的 DNA 中所残留的化学污染物不同。这会影响文库的制备效率和测序质量。请参阅牛津纳米孔社区的污染物 页面了解更多信息。
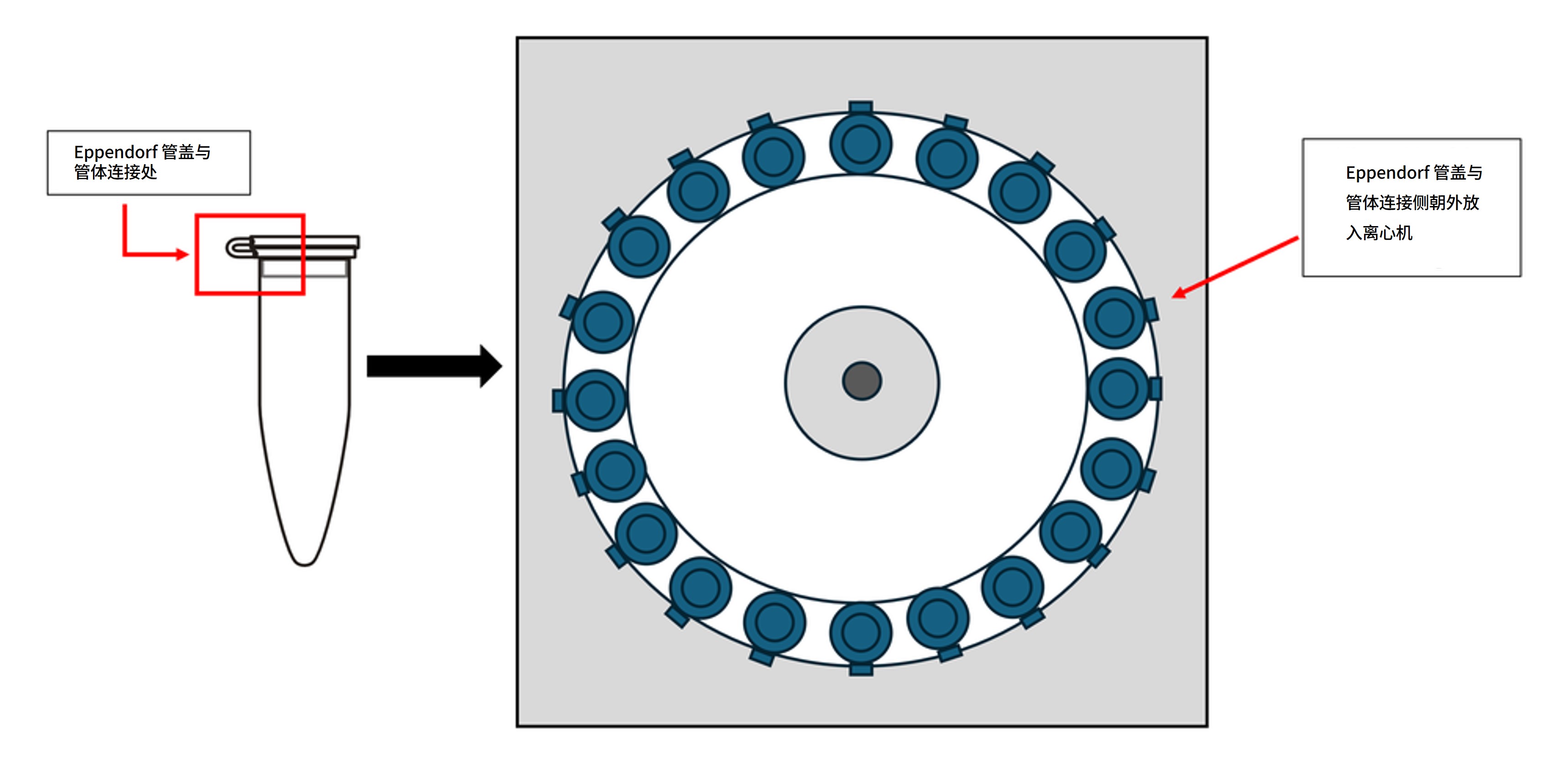
Eppendorf 离心管的放置方向
在所有离心步骤中,请将离心管盖与管体的连接处朝外放置,以便快速定位沉淀。
取出离心管时应轻拿轻放,避免扰动沉淀。
对于新用户,我们建议购买专供 Oxford Nanopore Technologies® 连接测序的 NEBNext® 配套模块 v2(NEB,E7672S 或 E7672L)。该配套模块内包含所有与连接测序试剂盒配套使用的 NEB 试剂。
尽管用户仍可使用早期版本的 NEBNext® 配套模块(NEB,E7180S 或 E7180L),但我们推荐的 v2 配套模块在 dA 尾添加和连接方面表现更优。
第三方试剂
Oxford Nanopore Technologies 推荐您使用本实验指南中列出的所有第三方试剂,并已对其进行验证。我们尚未对其它替代试剂进行测试。
我们建议您按制造商说明准备待用的第三方试剂。
测序芯片质检
我们强烈建议您在开始测序实验前,对测序芯片的活性纳米孔数进行质检。质检需在您收到 PromethION 测序芯片12周内进行。Oxford Nanopore Technologies 会对活性孔数量少于以下标准且尚未投入测序的芯片进行替换*:请您按照测序芯片质检文档中的说明进行芯片质检。
| 测序芯片 | 芯片上的活性孔数确保不少于 |
|---|---|
| PromethION 测序芯片 | 5000 |
*(请注意:自收到之日起,芯片须一直贮存于 Oxford Nanopore Technologies 推荐的条件下。且质检结果须在质检后的两日内递交给我们。)
为确保连接接头(LA)的高效连接,我们强烈建议使用连接测序试剂盒 V14 中提供的连接缓冲液(LNB),而非任何第三方连接酶缓冲液。
本试剂盒及其实验指南中使用的连接接头(LA)不可与其他测序接头互换使用。
连接测序试剂盒 V14 (SQK-LSK114)内容物
请注意: 本产品包含由贝克曼库尔特公司(Beckman Coulter, Inc)生产的 AMPure XP 试剂,并可与试剂盒一起于-20°C 下储存(试剂稳定性将不受损害)。
请注意: DNA 参照(DCS)是一段可比对至 Lambda 基因组 3' 端、且长度为 3.6 kb 的标准扩增子。
3. 使用 QIAGEN Puregene 细胞试剂盒进行人类细胞系 DNA 提取
材料
- 5x10⁶ cells(例如细胞培养物或组织样本)
耗材
- Puregene 细胞试剂盒(QIAGEN,158043)
- 新鲜配制并预冷的 70% 乙醇(以无核酸酶水配制)
- TE 缓冲液(10 mM Tris-HCl、1 mM EDTA、pH 8.0)(Fisher scientific,10224683)
- 磷酸盐缓冲盐溶液(PBS),pH 7.4(ThermoFisher,10010023)
- 异丙醇,100%(Fisher, 10723124)
- 15 ml Falcon离心管
- 2 ml Eppendorf DNA LoBind 离心管
- 1.5 ml Eppendorf DNA LoBind 离心管
- 接种环或一次性镊子
- Qubit dsDNA BR Assay(双链DNA宽范围检测)试剂盒(ThermoFisher ,Q32850)
- Qubit™ 分析管(Invitrogen, Q32856)
仪器
- 设定为 37°C 和 50°C 的培养箱或水浴锅
- 适用于 15 ml Falcon 管的离心机及转子
- 涡旋混匀仪
- 宽口移液枪头
- P1000 移液枪和枪头
- P200 移液枪和枪头
- 盛有冰的冰桶
- Qubit™ 荧光计(或用于质控检测的等效仪器)
在样本处理和建库过程中,建议使用宽口移液枪头,以避免 DNA 样本发生过度剪切。
混匀时应采用缓慢且温和的操作方式,以获得较长的 DNA 片段。但同时,请务必确保 DNA 与试剂充分混匀,混匀不足会导致读长和测序产出下降。
准备一支 1.5 ml Eppendorf DNA LoBind 管,加入约 1 ml 80% 乙醇,置于冰上预冷备用。
在 15 ml Falcon 管中,将 5 × 10⁶ 个细胞以 300 × g 离心 3 分钟收集沉淀。若细胞沉淀中仍有残留液体,可再次瞬时离心后,小心吸取残余上清液并丢弃。
注意: 所需细胞数量可能取决于样本类型。
向沉淀的细胞中加入 200 µl 1x PBS。
300 x g 离心三分钟。
注意避免扰动沉淀,用移液枪小心吸出上清液并弃去。
向沉淀的细胞中再次加入 200 µl 1× PBS,轻弹离心管或使用宽口枪头吹打以重悬沉淀。
细胞沉淀在重悬后应完全分散,以利于后续的细胞裂解步骤。
加入 3 ml 细胞裂解液(Cell Lysis Solution)。
注意: 使用宽口枪头轻柔吹打 10–15 次,直至样品完全均一、无结块。确保溶液混合均匀。
37℃ 下孵育 30 分钟。
注意: 孵育结束时,请确保溶液充分均一,且无结块残留。
您可按需使用宽口移液枪头吹打混匀,或轻柔倒转离心管以促进溶液混匀。
向样品中加入 5 µl RNase A 溶液,倒转混匀 20 次。
在 37°C 孵育 15 分钟,随后将样品置于冰上冷却 3 分钟。
向裂解液中加入 1000 µl 蛋白沉淀液(Protein Precipitation Solution),以最大转速涡旋震荡 5 秒 × 2 次。
2000 x g 离心 10 分钟。
蛋白应形成紧实的白色沉淀。
若蛋白沉淀不够紧实,可在冰上放置 5 分钟后重复离心。
在一支新的 15 ml Falcon 管中加入 3 ml 异丙醇。
将样品管中的上清液小心倒入装有异丙醇的 15 ml Falcon 管中。
倾倒过程中应确保蛋白沉淀保持完整,不被扰动。
__或者__,您也可使用移液枪转移上清液,操作过程中同样应确保蛋白沉淀保持完整,不被扰动。
注意: 如蛋白沉淀在任何步骤中被扰动,请重新以 2000 × g 离心 10 分钟。请确保仅转移澄清的上清液,防止蛋白污染最终洗脱液。
轻柔倒转离心管约 50 次混匀,直至 DNA 呈丝状或结块。
2000 × g 离心 Falcon 管 3 分钟。
DNA 可能在管底形成小的白色沉淀。
小心弃去上清液,并将离心管倒置在干净的吸水纸上以沥干残液。确保 DNA 沉淀未被扰动且留在管内。
注意: 上清液可通过移液或倾倒至吸水材料上去除。
操作时需小心,DNA 沉淀可能较为松散,容易脱落。
向样品中加入 300 μl 预冷、新鲜配制的 80% 乙醇,轻轻倒转数次以清洗 DNA 沉淀。
将 DNA 沉淀及全部 300 μl 乙醇转移至一支新的 1.5 ml Eppendorf 离心管中。
2000 × g 离心 1 分钟。
小心弃去上清液,并将离心管倒置在干净的吸水纸上以沥干残液。确保 DNA 沉淀未被扰动且留在管内。
注意: 上清液可通过移液或倾倒至吸水材料上去除。
操作时需小心,DNA 沉淀可能较为松散,容易脱落。
打开管盖,将 DNA 沉淀在空气中干燥 1 分钟。
注意: 请避免过度干燥,DNA 沉淀不应干燥至开裂。
向 DNA 沉淀中加入 200 μl TE 缓冲液(10 mM Tris-HCl、1 mM EDTA、pH 8.0)。轻弹离心管以重悬沉淀。
在 50°C 下孵育 2 小时,期间使用宽口枪头不时混匀全部体积(200 μl)。
注意: DNA 沉淀可能需要一定时间才能完全溶解;定量前请确保溶液已充分混匀。
可选: 亦可在室温下孵育过夜。
使用 Qubit dsDNA BR Assay(双链 DNA 宽范围检测)试剂盒对样品进行三次定量。 在继续后续步骤前,请确认三次测量结果一致。
注意: 以 5 × 10⁶ 个培养细胞为起始量,预计可获得约 20–35 µg 的 gDNA。
预期 Qubit 测定浓度约为 200–350 ng/μl。
如果 Qubit 测量结果存在较大差异,可能是由于 DNA 未充分重悬混匀所致。
此时建议延长孵育时间,以促进 DNA 完全溶解。
注意: 建议将样品置于恒温混匀仪中,在 50°C 条件下以 300 rpm 轻柔振荡孵育,以促进洗脱;亦可采用翻转式混匀仪。
提取的基因组 DNA(gDNA)可直接用于后续的片段大小筛选步骤,或在 4°C 条件下过夜保存。
4. 使用 Covaris g-TUBE™ 将 DNA 剪切至约 10 kb 作为建库起始材料
材料
- 2 µg 提取的高分子量基因组 DNA
耗材
- g-TUBE™(Covaris,520079)
- 无核酸酶水(如ThermoFisher,AM9937)
- 1.5 ml Eppendorf DNA LoBind 离心管
- (非必需)TE 缓冲液(10 mM Tris-HCl、0.1 mM EDTA,pH 8.0)
- Qubit dsDNA BR Assay(双链DNA宽范围检测)试剂盒(ThermoFisher ,Q32850)
- Qubit™ 分析管(Invitrogen, Q32856)
- Agilent 基因组 DNA 165 kb 分析试剂盒(Agilent,FP-1002-0275)
仪器
- 迷你离心机
- P200 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P2 移液枪和枪头
- Agilent Femto Pulse 系统(或用于读长质控的等效仪器)
- Qubit™ 荧光计(或用于质控检测的等效仪器)
如需长期保存文库,建议使用 TE 缓冲液(10 mM Tris-HCl、0.1 mM EDTA,pH 8.0),而非无核酸酶水。
将 2 μg 提取出的 gDNA 转移至至一洁净的 1.5 ml Eppendorf DNA LoBind 管中,并用无核酸酶水补足至 100 µl。
轻弹离心管充分混匀 DNA。使用迷你离心机瞬时离心。
将总体积为 100 µl 的基因组 DNA 样本转移至一Covaris g-TUBE™ 管中。
为获得约 10 kb 的片段长度,在室温下以 4300 × g 离心 g-TUBE™ 1 分钟。从离心机中取出 g-TUBE™ 管,并确认全部 DNA 溶液已流入 g-TUBE™ 管的下方。
注意: 请根据设备型号和起始样本选择合适的转速,以获得约 10 kb 的剪切片段。
如 DNA 仍停留在 g-TUBE™ 管的上方,则以相同速度再次离心 1 分钟。
倒转 g-TUBE™ 管,再次离心 1 分钟,收集片段化的 DNA。从离心机中取出 g-TUBE™ 管,并确认全部 DNA 溶液已流入 g-TUBE™ 管的下方。
如 DNA 仍停留在g-TUBE™ 管的上方,则以相同速度再次离心 1 分钟。
将 100 µl 片段化 DNA 转移至一支干净的 1.5 ml Eppendorf DNA LoBind 离心管中。
取 1 µl 片段化 gDNA,用 Qubit 定量,并使用 Femto-Pulse(Agilent)评估样本片段的长度。预期读长的 N50 应在 10 kb。
取 1 μg 样本用于后续文库构建,或将样本置于 4 °C 过夜保存。
5. DNA损伤及末端修复
材料
- 1 µg 片段化的基因组 DNA
- AMPure XP 磁珠(AXP)
耗材
- NEBNext®配套模块v2(NEB,E7672S或E7672L)中的NEBNext® FFPE DNA修复混合液
- NEBNext®配套模块v2(NEB,E7672S或E7672L)中的NEBNext® FFPE DNA修复缓冲液v2
- NEBNext®配套模块v2(NEB,E7672S或E7672L)中的NEBNext® Ultra II 末端修复酶混合物
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
- 无核酸酶水(如ThermoFisher,AM9937)
- 新制备的 80% 乙醇(用无核酸酶水配制)
- Qubit™ 分析管(Invitrogen, Q32856)
- 0.2 ml 薄壁PCR管
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- P1000 移液枪和枪头
- P100 移液枪和枪头
- P10 移液枪和枪头
- 迷你离心机
- 热循环仪
- Hula混匀仪(低速旋转式混匀仪)
- 磁力架
- 盛有冰的冰桶
可选仪器
- Qubit™ 荧光计(或用于质控检测的等效仪器)
测序芯片质检
我们建议您在开始文库制备之前,对测序芯片的活性纳米孔数量进行质检,以确保其足够支持实验的顺利进行。
更多信息,请参阅测序芯片质检文档 。
根据生产厂家说明制备 NEB 试剂,并置于冰上。
为获得最优表现,NEB建议如下:
1.于冰上解冻所有试剂。
2.轻弹或倒转试剂管,使其充分混匀。
注意: 请勿涡旋振荡 FFPE 修复混合液或 Ultra II 末端修复酶混合物。
3.每日首次打开一管试剂前,请务必先瞬时离心。
4.涡旋振荡 FFPE DNA 修复缓冲液 v2,确保充分混匀。
注意: 该缓冲液中可能含有白色沉淀。如发现沉淀,请待液体回复至室温后,使用移液枪上下吹打数次,打散沉淀;然后快速涡旋振荡混匀。
5.FFPE DNA 修复缓冲液 v2 可能呈淡黄色,此属正常现象,不影响其使用。
使用无核酸酶水配制 DNA 样本:
1.将来自样本提取步骤的 1 µg、10 kb 片段化 gDNA 转移至一支 0.2 ml 薄壁 PCR 管中。
2.如不足48 μl,请加入无核酸酶水补足。
3.吹打或轻弹离心管,充分混匀。
4.使用迷你离心机瞬时离心。
在装有 gDNA 的 0.2 ml 薄壁 PCR 管中,加入并混合以下试剂:
| 试剂 | 体积 |
|---|---|
| 前一步骤所得 DNA 样本 | 48 µl |
| NEBNext FFPE DNA 修复缓冲液 v2 | 7 µl |
| NEBNext FFPE DNA 修复混合液 | 2 µl |
| Ultra II 末端修复酶混合物 | 3 µl |
| 总体积 | 60 µl |
轻轻吹打以充分混匀,并瞬时离心。
使用热循环仪,在 20℃ 下孵育 5 分钟,然后在 65℃ 下孵育 5 分钟,随后在 4°C 保持。
涡旋振荡以重悬 AMPure XP 磁珠(AXP)。
将 DNA 样本转至干净的 1.5 ml Eppendorf DNA LoBind 离心管中。
将 60µl 重悬的 AMPure XP磁珠(AXP)加入 DNA 末端修复反应体系中,轻弹试管以充分混合。
将离心管置于 Hula 混匀仪(低速旋转式混匀仪)上室温孵育 5 分钟。
准备 600μl 新制备的 80% 乙醇(用无核酸酶水配制)。
将样品瞬时离心,并于磁力架上静置 10 分钟,待磁珠与液相分离,且液相澄清无色。保持离心管在磁力架上不动,用移液枪吸去清液。
保持试管在磁力架上不动,以 250 µl 新鲜制备的 80% 乙醇洗涤磁珠。小心不要扰动磁珠。用移液枪将乙醇吸走并弃掉。
重复上述步骤。
将离心管瞬时离心后置于磁力架上。用移液枪吸走残留的乙醇。让磁珠在空气中干燥约30秒,但不要干至表面开裂。
将离心管从磁力架上移开,轻轻吹打或轻弹离心管,使磁珠重悬于 61 µl 无核酸酶水中。室温下孵育 2 分钟。
将离心管静置于磁力架上至少 1 分钟,直到磁珠和液相分离,且洗脱液澄清无色。
将 61µl 洗脱液转移至一支新的 1.5ml Eppendorf DNA LoBind 管中。
取1µl洗脱样品,用Qubit定量。
经损伤修复及末端预处理后的 DNA 可直接用于接头连接;必要时可在 4°C 条件下过夜保存。
6. 接头连接及纯化
材料
- 连接接头(LA)
- 连接测序试剂盒内的连接缓冲液(LNB)
- 长片段缓冲液(LFB)
- AMPure XP 磁珠(AXP)
- 连接测序试剂盒中提供的洗脱缓冲液(EB)
耗材
- 耐盐T4 DNA连接酶(NEB, M0467)
- 1.5 ml Eppendorf DNA LoBind 离心管
- Qubit™ 分析管(Invitrogen, Q32856)
- Qubit dsDNA HS Assay(双链DNA高灵敏度检测)试剂盒(Invitrogen, Q32851)
仪器
- Hula混匀仪(低速旋转式混匀仪)
- 磁力架
- 迷你离心机
- 涡旋混匀仪
- P1000 移液枪和枪头
- P100 移液枪和枪头
- P20 移液枪和枪头
- P10 移液枪和枪头
- Qubit™ 荧光计(或用于质控检测的等效仪器)
虽然第三方连接酶产品可能附带缓冲液,但连接测序试剂盒中提供的连接缓冲液(LNB)在连接接头(LA)时效率更高。
瞬时离心连接接头(LA)和耐盐 T4® DNA连接酶,置于冰上。
于室温下解冻连接缓冲液(LNB),解冻后瞬时离心,并用移液枪吹打混匀。该缓冲液的黏度较高,涡旋振荡会很难混匀。解冻并混匀后,立即置于冰上。
将洗脱缓冲液(EB)于室温下解冻,涡旋振荡混匀后,瞬时离心后置于冰上。
于室温下解冻一管长片段缓冲液(LFB),涡旋振荡混匀,瞬时离心后置于冰上。
在一支1.5ml Eppendorf DNA LoBind离心管内,将所有试剂按以下顺序混合:
每添加一样试剂后,请吹打混匀10-20次,再添加下一样试剂。
| 试剂 | 体积 |
|---|---|
| 前一步骤所得DNA样品 | 60 µl |
| 连接接头(LA) | 5 µl |
| 连接缓冲液(LNB) | 25 µl |
| 耐盐 T4® DNA连接酶 | 10 µl |
| 总体积 | 100 µl |
轻轻吹打以充分混匀,并瞬时离心。
室温下孵育10分钟。
涡旋振荡以重悬 AMPure XP 磁珠(AXP)。
将 40µl 重悬的 AMPure XP(AXP)磁珠加入反应体系中,轻弹离心管以充分混合。
将离心管置于Hula混匀仪(低速旋转式混匀仪)上室温孵育5分钟。
将样品瞬时离心后置于磁力架上,待磁珠与液相完全分离,且液相澄清无色。保持离心管在磁力架上不动,用移液枪吸去清液。
加入 250 μl 长片段缓冲液(LFB)洗涤磁珠。轻弹离心管将磁珠混匀后,将离心管瞬时离心,再放回磁力架,静置至少 5 分钟,待磁珠和液相分离。保持离心管在磁力架上不动,用移液枪吸去清液。
请注意: 吸取上清液时请谨慎操作,由于缓冲液黏度较高,可能会将磁珠一同吸出。
重复上述步骤。
将离心管瞬时离心后置于磁力架上。用移液枪吸走残留的上清液。让磁珠在空气中干燥约 30 秒,但不要干至表面开裂。
将离心管从磁力架上移开。将磁珠重悬于 33 µl 洗脱缓冲液中(EB)。瞬时离心,然后在 37°C 下孵育10分钟。
将离心管置于磁力架上 10 分钟,直至磁珠和液相分离,且洗脱液澄清无色。
将此 33 µl 洗脱液转移至一支新的 1.5ml Eppendorf DNA LoBind 管中。
将磁珠丢弃。
取 1µl 洗脱样品,用 Qubit 定量。
对于 N50 为 10 kb 的文库,我们建议您向 R10.4.1 测序芯片中加入不少于 200–300 ng(35–50 fmol) 的最终制备好的文库。
本方案经过优化,可实现单个基因组约 30× 覆盖度的测序分析。
为在测序初期最大限度提高纳米孔利用率,请确保上样至测序芯片的 DNA 片段长度约为 10 kb,且上样量为 200–300 ng。测序初期纳米孔利用率偏低会增加孔道末端堵塞的发生概率,从而降低测序产出。
您可按需使用质量与摩尔数转换计算器,如 NEB 计算器。
构建好的文库即可用于测序芯片上样。在上样前,请将文库置于冰上或4℃条件下保存。
文库保存建议
若为 短期 保存或重复使用(例如在清洗芯片后再次上样),我们建议将文库置于Eppendorf LoBind 离心管中 4℃ 保存。 若为一次性使用且储存时长 超过3个月 ,我们建议将文库置于Eppendorf LoBind 离心管中 -80℃ 保存。
7. PromethION测序芯片的预处理及上样
材料
- 测序缓冲液(SB)
- 文库颗粒(LIB)
- 文库溶液(LIS)
- 测序芯片系绳(FCT)
- 测序芯片冲洗液(FCF)
耗材
- PromethION 测序芯片
- 1.5 ml Eppendorf DNA LoBind 离心管
仪器
- PromethION 测序设备
- PromethION 测序芯片遮光片
- P1000 移液枪和枪头
- P200 移液枪和枪头
- P20 移液枪和枪头
本试剂盒仅兼容R10.4.1测序芯片(FLO-PRO114M)。
将芯片从冰箱中取出后,请将其置于室温环境孵育 20 分钟再插入 PromethION 测序仪。潮湿环境下的测序芯片上可能会形成冷凝水。因此,请检查测序芯片顶部和底部的金色连接器引脚处是否有水凝结。如有,请使用无绒布擦干。请确保测序芯片的底部有热垫(黑色)覆盖。
于室温下解冻测序缓冲液(SB)、文库颗粒(LIB)或文库溶液(LIS)、测序芯片系绳(FCT)和测序芯片冲洗液(FCF)。完全解冻后,涡旋振荡混匀。然后瞬时离心,置于冰上。
按下文制备测序芯片的预处理液,室温下涡旋振荡混匀。
请根据待上样测序芯片的数量,另拿一支适当体积的离心管,按下表制备测序芯片预处理液。
| 试剂 | 体积(每张芯片) |
|---|---|
| 测序芯片冲洗液 (FCF) | 1,170 µl |
| 测序芯片系绳(FCT) | 30 µl |
| 总体积 | 1,200 µl |
对 PromethION 2 Solo,请按以下步骤为测序芯片上样:
1.将测序芯片平放在金属板上。
2.将测序芯片推入对接端口,直至金色引脚或绿色电路板不可见。

对PromethION 24/48,将测序芯片插入相应卡槽的对接端口:
1.将测序芯片与连接器横竖对齐,以便顺利卡入。
2.用力下压芯片至卡槽,并确认卡夹位置归位。


如插入测序芯片的角度出现偏差,可能会损坏 PromethION 上的引脚并影响测序结果。如您发现 PromethION 测序仪芯片卡槽上的引脚损坏,请通过电子邮件(support@nanoporetech.com)联系我们的技术支持团队。

为文库上样前,完成测序芯片质检,查看可用孔数目。
如此前已对测序芯片进行过质检,则此步骤可省略。
更多信息,请参阅测序芯片质检文档。
顺时针滑动加液孔孔盖,将其打开。

小心地从测序芯片中反旋吸出缓冲液。请勿吸出超过 20-30 µl的缓冲液,并确保芯片上的纳米孔阵列一直有缓冲液覆盖。将气泡引入阵列会对纳米孔造成不可逆转地损害。
在加液孔打开的状态下,按下述步骤吸取少量液体,同时避免引入气泡:
1.将 P1000 移液枪转至 200µl 刻度。
2.将枪头垂直插入加液孔中。
3.反向转动移液枪量程调节转纽,直至移液枪刻度在 220-230 µl之间,或直至您看到有少量缓冲液进入移液枪枪头。

向芯片的加液孔中加入 500 µl 芯片预处理液。加入过程中,请避免引入气泡。等待5分钟。与此同时,您可按以下步骤准备上样文库。

将含有文库颗粒的LIB管用移液枪吹打混匀。
LIB管内的文库颗粒分散于悬浮液中。由于颗粒沉降速度非常快,因此请在混匀颗粒后立即使用。
对于大多数测序实验,我们建议您使用文库颗粒(LIB)。但如文库较为粘稠,您可考虑使用文库溶液(LIS)。
在一支新的1.5ml Eppendorf DNA LoBind离心管内,将所有试剂按以下顺序混合:
| 试剂 | 每张测序芯片的上样体积 |
|---|---|
| 测序缓冲液(SB) | 100 µl |
| 文库颗粒 (LIB),使用前充分混匀;或文库溶液 (LIS) | 68 µl |
| DNA 文库 | 32 µl |
| 总体积 | 200 µl |
请注意: 为提高纳米孔阵列的覆盖度,已适当增加文库上样体积。
缓慢向芯片的加液口中加入500 µl预处理液,完成芯片的预处理。

临上样前,用移液枪轻轻吹打混匀制备好的文库。
使用 P1000 移液枪向加液孔中加入 200 µl 文库。

合上加液孔孔盖。

为获得最佳测序产出,在文库样本上样后,请立即在测序芯片上安装遮光片。
我们建议在清洗芯片并重新上样时,将遮光片保留在测序芯片上。一旦文库从测序芯片中吸出,即可取下遮光片。
如遮光片不在测序芯片上,请您按照以下步骤安装:
1.将遮光片的中空部分(空槽)与测序芯片的加液孔孔盖对齐。确保遮光片的前沿位于测序芯片ID的上方。
2.用力下压遮光片的卡垫部分,遮光片空槽边缘会随卡垫卡入加液孔孔盖下方。


准备就绪后,合上 PromethION 设备上盖。
请在为 PromethION 芯片上样后,等待 10 分钟再启动实验,以提高芯片产出。
8. 数据采集和碱基识别
如何开始测序
MinKNOW 软件负责仪器控制,数据采集和实时碱基识别。
我们建议在测序过程中使用高精准(HAC)碱基识别模式进行实时碱基识别,并在 P2 Solo 或 P24 / P48 设备上将 BAM 设为输出文件格式。
运行 wf-human-variation 工作流程时,需提供由测序生成的 BAM 文件作为输入数据。
请参阅以下链接,获取有关设备设置和测序运行的详细操作说明:
PromethION 24 及 48:"Starting a sequencing run with PromethION 24 and 48” (使用 PromethION 24 与 48 进行测序)
PromethION 2 Solo:"Starting a sequencing run on PromethION 2 Solo"((使用 PromethION 2 Solo 进行测序))
下文列出了推荐的 MinKNOW 测序参数设置。
PromethION 平台人类 10 kb 样本的 MinKNOW 参数设置
我们建议您在设置 MinKNOW 测序实验时,启用修饰碱基识别选项,并确保选择 BAM 作为输出格式。其余测序参数保持默认设置即可。 MinKNOW 设置建议如下:
测序芯片位置
测序芯片位置 : [用户定义]
实验名称 : [用户定义]
测序芯片类型 : FLO-PRO114M
样本编号 :[用户定义]
试剂盒
试剂盒选择 :连接测序试剂盒(SQK-LSK114)
运行配置
测序及分析
碱基识别 :开[默认] 修饰碱基 :开并选择“CG位点 5mC 和 5hmC” 模型 :高精准碱基识别工具(HAC)[默认]
条码拆分 :关 [默认]
比对 :关 [默认]
鉴于实时比对可能降低系统处理性能,目前不建议在测序过程中启用。
适应性采样 :关 [默认]
进阶选项 活性通道选择 :开 [默认] 孔扫描间隔时间 :1.5 [默认] 预留孔 :开 [默认]
数据目标
运行限制条件 :72 小时 [默认]
输出
输出文件的格式 .POD5 :开 [默认] .FASTQ :开 [默认] .BAM :开
过滤 :开 [默认] 质量值 :9 [默认] 最短读长 :200 bp [默认]
我们不建议在测序过程中启用实时比对,以免降低系统处理速度。
您可在测序完成后,采用以下任一方法对 BAM 文件进行比对:
| 在 MinKNOW 中对 BAM 文件进行比对 | 在 wf-human-variation 工作流程中对 BAM 文件进行比对 |
|---|---|
| 在 MinKNOW 完成实时碱基识别后再对生成的 BAM 文件进行比对,以防止系统处理速度下降。 比对后的 BAM 文件可作为 wf-human-variation 工作流程的输入文件。 使用经过比对的 BAM 文件作为输入时,工作流程的运行时间约为 1–2 小时。 | 在配置 wf-human-variation 工作流程时,可同时提供参考基因组和未经比对的 BAM 文件。 使用未比对 BAM 文件作为输入时,工作流程的运行时间约为 5–8 小时。 |
更多信息,请参阅本指南的下游分析部分。
9. 下游分析
人类细胞 DNA 序列数据的分析
我们推荐您使用wf-human-variation 工作流程对人类细胞 DNA 测序数据进行分析。该端到端分析流程基于 Nextflow 构建,可用于单核苷酸多态性(SNP)和结构变异(SV)的检测,并报告 DNA 甲基化信息。
建议使用由 MinKNOW 生成的 BAM 文件(碱基识别时选择修饰碱基模型)作为 wf-human-variation 工作流程的输入。若未在 MinKNOW 中执行测序序列与参考基因组的比对,建议运行 wf-basecalling 工作流程进行碱基识别,并通过 --output_bam 参数将结果保存为 BAM 格式文件。
下文列出了该分析工作流程中使用的工具。这些工具既可独立使用,也可组合使用。
1.Sniffles2 用于检测结构变异(SV),输出结果包括一份 HTML 格式的质控(QC)报告,以及一份包含检测到的变异及其质量分数的 VCF 文件。
2.Clair3 用于检测单核苷酸多态性(SNP),输出结果包括一份 HTML 格式的质控(QC)报告,以及一份包含检测到的变异及其质量分数的 VCF 文件。
3.modkit 用于从 BAM 文件中提取 DNA 甲基化信息,并生成 BEDmethyl 格式的汇总文件。
wf-human-variation 工作流程已预设适当参数,通常仅需针对参考基因组和 Clair3 模型的选择进行调整。更多详细信息请参阅该项目的官方文档。
wf-human-variation 工作流的结果可在基因组浏览器(如 IGV)中直观查看,并通过三级分析软件评估变异的已知致病性。
EPI2ME 分析流程
wf-human-variation 工作流程基于 Nextflow 软件运行。
我们建议您使用 EPI2ME 进行下游分析。EPI2ME 提供图形化用户界面,可运行 Nextflow 工作流程,帮助用户高效开展生物信息学分析。平台内的分析流程由长读长测序领域的专家精心开发维护。点击此处,查看我们当前提供的全部 EPI2ME 工作流程。
新用户可参考快速入门指南,其中详细介绍了 EPI2ME 界面的使用方法。
在 EPI2ME 平台上运行 wf-human-variation 工作流程所需的计算资源
| 推荐配置 | 最低配置 |
|---|---|
| CPUs = 32 | CPUs = 16 |
| 内存 = 128GB | 内存 = 32GB |
__预计运行时间__:运行时间取决于测序类型(靶向测序或全基因组测序)、测序覆盖度以及所选择的具体分析功能。例如,使用推荐的计算资源时,对一个覆盖度为 90× 的人类样本同时开展多项变异分析(--snp --sv --mod --str --cnv --phased --sex male),整个分析流程可在 8 小时内完成。
__是否支持ARM 处理器__:否
wf-human-variation 工作流也支持通过命令行界面(CLI)运行。
更多信息请参阅相关的GitHub 页面 。
注意: 命令行界面(CLI)仅推荐具备相关经验的用户使用。
点击桌⾯快捷⽅式打开 EPI2ME 应用程序。
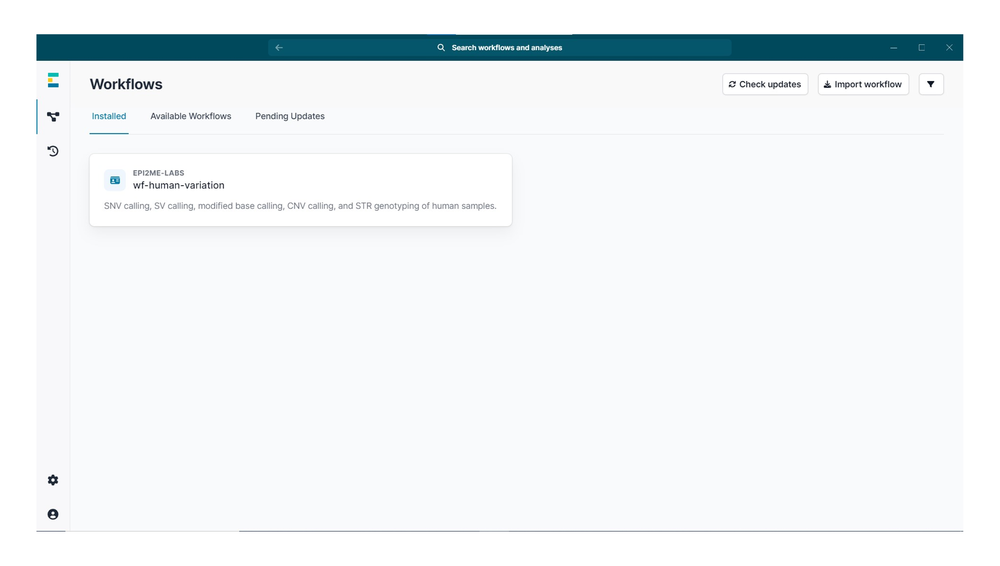
在 EPI2ME 首页,打开左侧边栏中的“Workflow”(工作流程)选项卡。

切换至“Available workflows”(可用的工作流程)选项卡,点击 wf-human-variation 工作流程。

点击“install”(安装)/

安装完成后,切换至“Installed”(已安装) 选项卡,点击已安装的 wf-human-variation 工作流。
如已安装该工作流程,请点击“Update workflow”(更新工作流程)以检查并更新至最新版本。
我们建议您使用最新版本的工作流程,已获得最佳分析结果。

单击“Run this workflow”(运行此工作流程)以打开启动引导程序。
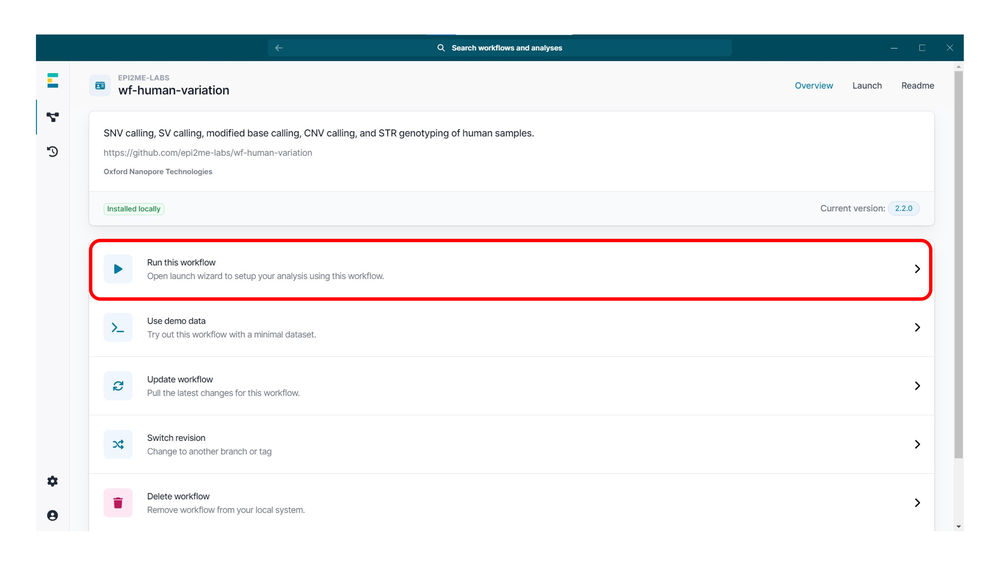
选择运行该工作流程的环境:

在 wf-human-variation 分析中,点击选择需要运行的子工作流程。
请在 “Main options(主要选项)” 选项卡中设置 “Sample name”(样本名称),以区分并标识工作流程的输出结果。

wf-human-variation 工作流程支持输入 BAM 格式的测序数据,包括单个 BAM 文件或包含多个 BAM 文件的文件夹。
经过比对或未经比对的 BAM 文件均可作为输入:
| 在 MinKNOW 中对 BAM 文件进行比对(在运行 wf-human-variation 之前) | 在运行 wf-human-variation 工作流程的过程中对 BAM 文件进行比对 |
|---|---|
| 在 MinKNOW 完成实时碱基识别后再对生成的 BAM 文件进行比对,以防止系统处理速度下降。 比对后的 BAM 文件可作为 wf-human-variation 工作流程的输入文件。 有关在 MinKNOW 中进行本地比对(post-run alignment)的更多信息,请参阅MinKNOW 实验指南。 使用经过比对的 BAM 文件作为输入时,工作流程的运行时间约为 1–2 小时。 | 在配置 wf-human-variation 工作流程时,可同时提供参考基因组和未经比对的 BAM 文件。 使用未比对 BAM 文件作为输入时,工作流程的运行时间约为 5–8 小时。 |
在“Main options(主要选项)”中上传测序数据,数据格式可为单个 BAM 文件或包含多个 BAM 文件的文件夹。

如果输入的 BAM 文件未经比对,请在“Main options(主要选项)”中上传 FASTA 格式的参考基因组。

点击“Lauch workflow”(启动工作流程)。
请确保所有参数选项前均显示绿色对勾。

wf-human-variation 工作流程完成后,将生成分析报告及相关输出文件。
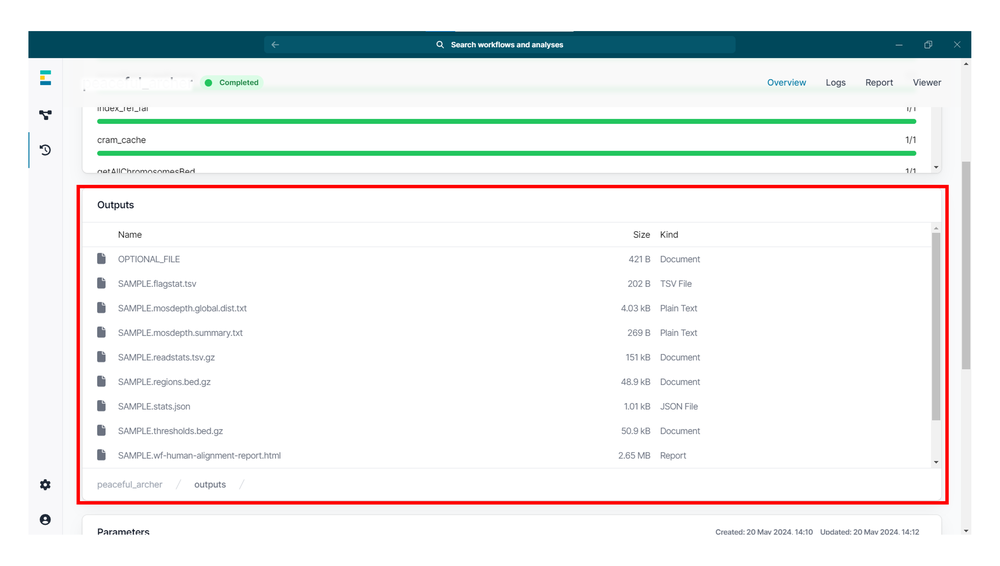
wf-human-variation 工作流程输出
该工作流程的 主要输出文件 包括:
- 通过
--snp生成的 gzip 压缩 VCF 文件,包含样本中的 SNP 变异信息 - 通过
--sv生成的 gzip 压缩 VCF 文件,包含样本中的结构变异(SV)信息 - 通过
--mod生成的 gzip 压缩 bedMethyl 文件,汇总修饰碱基的计数结果 - HTML 报告,展示工作流程的主要分析结果,包括质控指标以及 SNP 和 SV 的检测情况
- 若输入的 BAM 文件未经比对,工作流程将生成一份 CRAM 文件,其中包含用于下游变异检测的比对结果
工作流程的 次级输出文件 包括:
运行
mosdepth,会输出以下文件:{sample_name}.mosdepth.global.dist.txt:针对单个及全部参考序列,显示总碱基数比例的累积分布{sample_name}.regions.bed.gz:所提供 BED 文件中每个区域的平均覆盖度{sample_name}.thresholds.bed.gz:每个区域中,覆盖度达到或超过各阈值(1X, 10X, 20X, 30X)的碱基数
bamstats 输出文件包括:
{sample_name}.readstats.tsv.gz:由 bamstats 生成的、经过 gzip 压缩的 TSV 文件,汇总了逐条比对(per-alignment)的统计信息{sample_name}.ftagstat.tsv:文本文件,提供针对每个参考序列的比对统计摘要信息
wf-human-variation 工作流程使用提示
通过指定 --phased 选项,可对 SNP、结构变异(SV)以及碱基修饰进行分相分析。
为提高结构变异检测的准确性,建议通过 --tr_bed 参数提供与所用参考基因组版本匹配的串联重复 BED 文件。
如需使用 --mod 参数汇总甲基化结果,请在碱基识别阶段选用支持碱基修饰识别的模型,以确保生成的 BAM 文件中包含 MM 和 ML 标签。在 MinKNOW 中进行碱基识别时,请在碱基识别设置中勾选“Modified bases(修饰碱基)”选项,并选择“5mC”模型。
在进行碱基识别或序列比对时,请务必保留所使用的参考基因组文件;否则,CRAM 文件在缺少对应参考序列的情况下将无法读取。
如需查看全部可用的碱基识别模型,请参阅 Dorado 官方文档。
10. 测序芯片的重复利用及回收
材料
- 测序芯片清洗剂盒(EXP-WSH004)
完成测序实验后,如您希望再次使用测序芯片,请按照“测序芯片清洗试剂盒实验指南”进行操作,并将清洗后的芯片置于 +2 至 +8℃ 保存。
您可在纳米孔社区获取测序芯片清洗试剂盒实验指南。
我们建议您在停止测序实验后尽快清洗测序芯片。如若无法实现,请将芯片留在测序设备上,于下一日清洗。
或者,请按照回收程序将测序芯片返还至 Oxford Nanopore。
您可在 此处找到回收测序芯片的说明。
如果您遇到问题或对测序实验有疑问,请参阅本实验指南在线版本中的“疑难解答指南”一节。
11. DNA提取和文库制备过程中可能出现的问题
以下表格列出了提取和文库制备过程中的常见问题,以及可能的原因和解决方法。
我们还在 Nanopore 社区的Support板块提供了常见问题解答(FAQ)。
如果以下方案仍无法解决您的问题,请通过电邮(support@nanoporetech.com)或 纳米孔社区的在线支持(LiveChat)联系我们。
低质量样本
经AMPure磁珠纯化后的DNA回收率低
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 低回收率 | AMPure磁珠量与样品量的比例低于预期,导致DNA因未被捕获而丢失 | 1.AMPure 磁珠沉降速度较快,因此在将磁珠加入样品前,请务必充分重悬混匀。 2.当AMPure磁珠量与样品量的比值低于0.4:1时,所有的DNA片段都会在纯化过程中丢失。 |
| 低回收率 | DNA片段短于预期 | AMPure磁珠量与样品量的比值越低,针对短片段的筛选就越严格。每次实验时,请先使用琼脂糖凝胶(或其他凝胶电泳方法)确定起始DNA的长度,据此计算出合适的AMPure磁珠用量。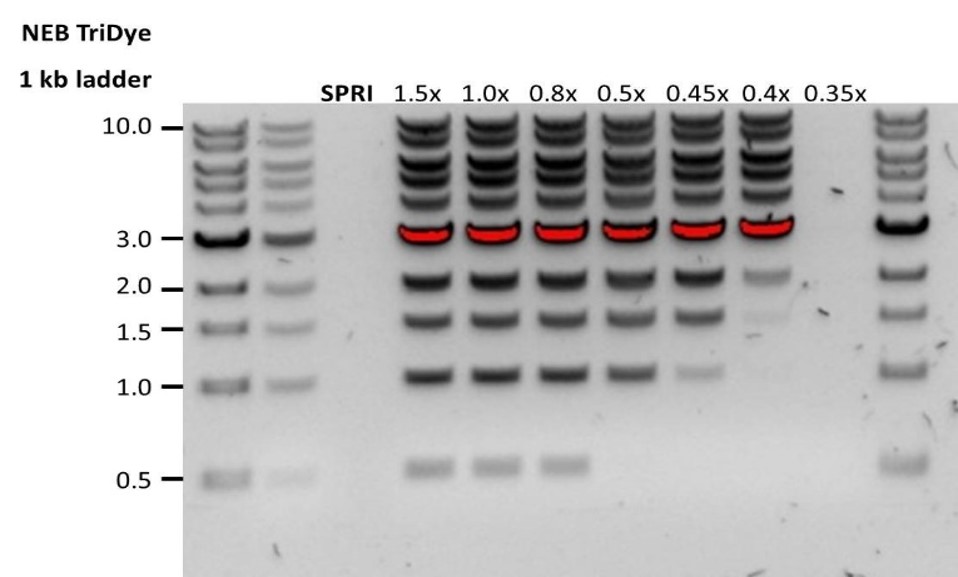 |
| 末端修复后的DNA回收率低 | 清洗步骤所用乙醇的浓度低于70% | 当乙醇浓度低于70%时,DNA会从磁珠上洗脱下来。请确保使用正确浓度的乙醇。 |
12. 测序过程中可能出现的问题
以下表格列出了提取和文库制备过程中的常见问题,以及可能的原因和解决方法。
我们还在 Nanopore 社区的Support板块提供了常见问题解答(FAQ)。
如果以下方案仍无法解决您的问题,请通过电邮(support@nanoporetech.com)或 纳米孔社区的在线支持(LiveChat)联系我们。
MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数 | 纳米孔阵列中引入了气泡 | 在对通过质控的芯片进行预处理之前,请务必排出预处理孔附近的气泡。否则,气泡会进入纳米孔阵列对其造成不可逆转地损害。如何为 PromethION 测序芯片的视频中演示了避免引入气泡的最佳操作方法。 |
| MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数 | 测序芯片没有正确插入测序仪 | 停止测序,将芯片从测序仪中取出,再重新插入测序仪内。请确保测序芯片牢固嵌入测序仪中,并已到达目标温度。如用户使用的是GridION/PromethION测序仪,也可尝试将芯片插入仪器的其它芯片槽进行测序。 |
| MinKNOW Mux 扫描在测序起始时报告的活性孔数少于芯片质检时报告的活性孔数 | 文库中残留的污染物对纳米孔造成损害或堵塞 | 在测序芯片质检阶段,我们用芯片储存缓冲液中的质控DNA分子来评估活性纳米孔的数量。而在测序开始时,我们使用DNA文库本身来评估活性纳米孔的数量。因此,活性纳米孔的数量在这两次评估中会有约10%的浮动。如测序开始时报告的孔数明显降低,则可能是由于文库中的污染物对膜结构造成了损坏或将纳米孔堵塞。用户可能需要使用其它的DNA/RNA提取或纯化方法,以提高起始核酸的纯度。您可在 污染物专题技术文档中查看污染物对测序实验的影响。请尝试其它不会导致污染物残留的 提取方法。 |
MinKNOW脚本失败
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| MinKNOW显示 "Script failed”(脚本失败) | 重启计算机及MinKNOW。如问题仍未得到解决,请收集 MinKNOW日志文件并联系我们的技术支持。如您没有其他可用的测序设备,我们建议您先将装有文库的测序芯片置于4°C 储存,并联系我们的技术支持团队获取进一步储存上的建议。 |
纳米孔利用率低于40%
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 纳米孔利用率<40% | 测序芯片中的文库量不够 | 在人类基因组测序方案中,应向 R10.4.1 测序芯片加载 200–300 ng 的高质量文库,以保持较高的纳米孔利用率。 |
| 纳米孔利用率接近0 | 使用连接测序试剂盒,但接头并未与DNA成功连接 | 请确保您在“测序接头连接”步骤中使用的是 NEBNext 快速连接模块(E6056),以及 SQK-LSK114 试剂盒中的连接缓冲液(LNB)。同时,请确保每种试剂的用量正确。您可通过制备Lambda对照文库来检验第三方试剂的可用性。 |
| 纳米孔利用率接近0 | 使用连接测序试剂盒;但在接头连接后的纯化步骤中并未使用LFB 或SFB洗涤,而是使用了酒精 | 酒精可导致测序接头上的马达蛋白变性。请确保在测序接头连接后使用洗涤缓冲液(LFB或SFB)。 |
| 纳米孔利用率接近0 | 测序芯片中无系绳 | 系绳(FCT 管)随测序芯片预处理液加至芯片。请确保在制备预处理液时,将测序芯片系绳(FCT)加入测序芯片冲洗液(FCF)中。 |
读长短于预期
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 读长短于预期 | DNA样本降解 | 读长反映了起始DNA片段的长度。起始DNA在提取和文库制备过程中均有可能被打断。 1.请查阅纳米孔社区中的 提取方法 以获得最佳DNA提取方案. 2.在进行文库制备之前,请先跑电泳,查看起始DNA片段的长度分布。 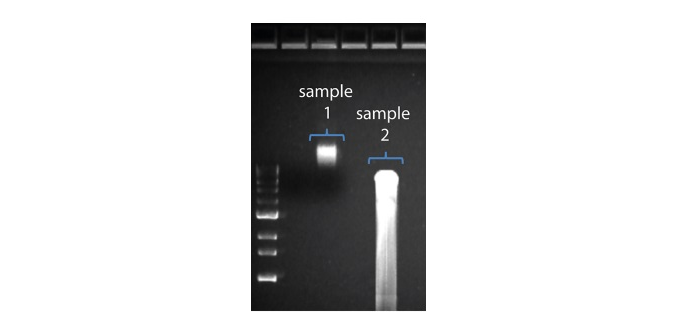 在上图中,样本1为高分子量DNA,而样本2为降解样本。 在上图中,样本1为高分子量DNA,而样本2为降解样本。3.在制备文库的过程中,请避免使用吹打或/和涡旋振荡的方式来混合试剂。轻弹或上下颠倒离心管即可。 |
大量纳米孔处于不可用状态
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
大量纳米孔处于不可用状态 (在通道面板和纳米孔活动状态图上以蓝色表示) 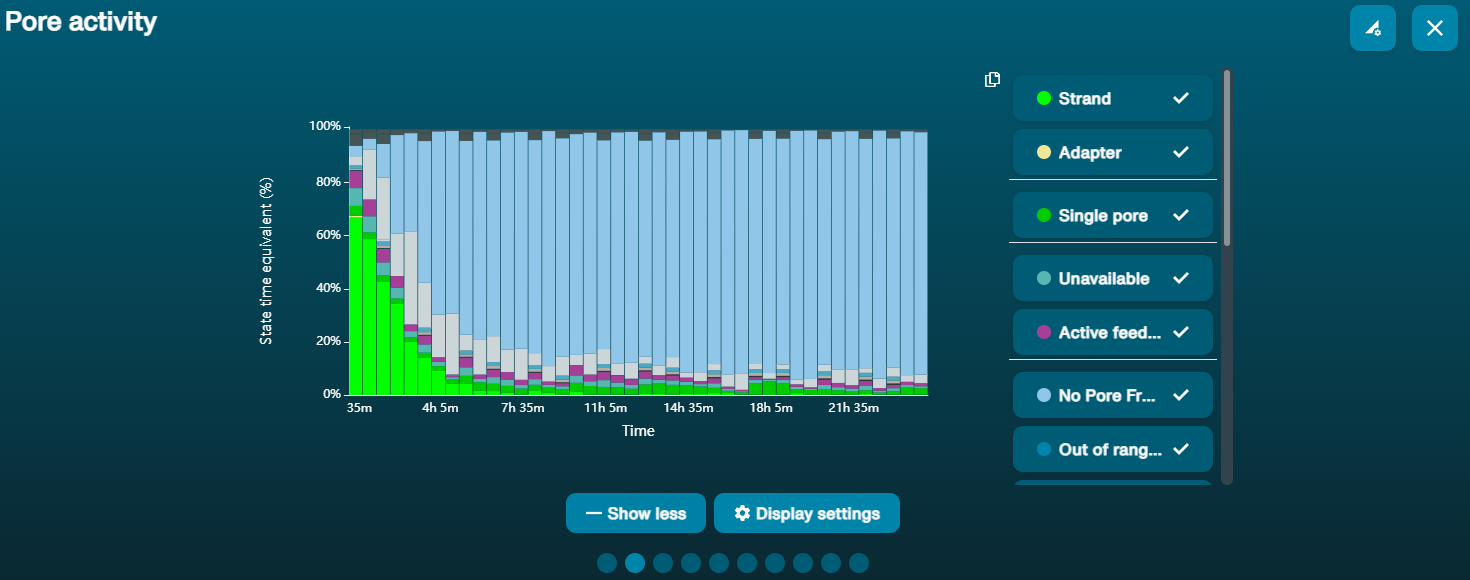 上方的纳米孔活动状态图显示:状态为不可用的纳米孔的比例随着测序进程而不断增加。 上方的纳米孔活动状态图显示:状态为不可用的纳米孔的比例随着测序进程而不断增加。 | 样本中含有污染物 | 使用MinKNOW中的“Unblocking”(疏通)功能,可对一些污染物进行清除。如疏通成功,纳米孔的状态会变为"测序孔"(sequencing pore)。若疏通后,状态为不可用的纳米孔的比例仍然很高甚至增加: 1.用户可使用 测序芯片冲洗试剂盒 (EXP-WSH004)进行核酸酶冲洗 操作,或 2.使用PCR扩增目标片段,以稀释可能导致问题的污染物。 |
大量纳米孔处于“失活”(Inactive)状态
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 大量纳米孔处于失活状态(在通道面板和纳米孔活动状态图上以浅蓝色表示。膜结构或纳米孔遭受不可逆转地损伤 | 测序芯片中引入了气泡 | 芯片预处理和文库上样过程中引入的气泡会对纳米孔带来不可逆转地损害。请观看如何为 PromethION 测序芯片上样了解最佳操作方法。 |
| 大量纳米孔处于失活/不可用状态 | 存在与DNA共纯化的化合物 | 已知的化合物包括多糖等。 1.使用 QIAGEN PowerClean Pro 试剂盒进行纯化。 2.利用QIAGEN REPLI-g 试剂盒对原始 gDNA 样本进行全基因组扩增。 |
| 大量纳米孔处于失活/不可用状态 | 样本中含有污染物 | 您可在Contaminants 中查看污染物对测序实验的影响。请尝试其它不会导致污染物残留的提取方法。 |
温度波动
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| 温度波动 | 测序芯片和仪器接触不良 | 检查芯片背面的金属板是否有热垫覆盖。重新插入测序芯片,用力向下按压,以确保芯片的连接器引脚与测序仪牢固接触。如问题仍未得到解决,请联系我们的技术支持。 |
未能达到目标温度
| 现象 | 可能原因 | 措施及备注 |
|---|---|---|
| MinKNOW显示“未能达到目标温度” | 测序仪所处环境低于标准室温,或通风不良(以致芯片过热) | MinKNOW会限定测序芯片达到目标温度的时间。当超过限定时间后,系统会显示出错信息,但测序实验仍会继续。值得注意的是,在错误温度下测序可能会导致通量和数据质量(Q值)的降低。请调整测序仪的摆放位置,确保将其置于室温下、通风良好的环境中,再在MinKNOW中重启进程。 |









