Flow Cell Wash Kit (Q-EXP-WSH004) (Q_WSH_revB_26Sep2024)
GridION: Protocol
Flow Cell Wash Kit (Q-EXP-WSH004) V Q_WSH_revB_26Sep2024
For Research Use Only
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
Washing a flow cell
概要
For Research Use Only
1. Overview of the protocol
Introduction to the Flow Cell Wash Kit
The Flow Cell Wash Kit allows sequential runs of multiple sequencing libraries on the same flow cell. It works by removing the first library and refreshing the system ready for a subsequent library to be loaded. This procedure provides the opportunity to utilise the same flow cell a number of times, maximising the available run time, particularly for cases where less data per library is required. Following the wash step, Storage Buffer can be introduced into the flow cell, allowing storage of the flow cell before subsequent library additions. The Flow Cell Wash Kit is compatible with Q-Line MinION Flow Cells.
Please note, although the wash procedure should remove 99.9% of the library, some residual DNA may remain on the flow cell. For this reason, you may prefer to barcode your libraries when used in conjunction with the Flow Cell Wash Kit, so that reads from different libraries can be separated from each other. Successful deconvolution of DNA reads has been demonstrated in Oxford Nanopore's internal development.
2. Equipment and consumables
材料
- Flow Cell Wash Kit (Q-EXP-WSH004)
装置
- P1000 ピペット及びチップ
- P20 ピペットとチップ
- アイスバケツ(氷入り)
Flow Cell Wash Kit contents (Q-EXP-WSH004)
| Contents | Volume (µl) | No. of tubes | No. of uses |
|---|---|---|---|
| Wash Mix (WMX) | 15 | 1 | 6 |
| Wash Diluent (DIL) | 1,300 | 2 | 6 |
| Storage Buffer (S) | 1,600 | 2 | 6 |
- Wash Mix (WMX) contains DNase I.
- Wash Diluent (DIL) contains the exonuclease buffer that maximises activity of the DNase I.
- The Storage Buffer allows flow cells to be stored for extended periods of time.
3. Washing a MinION Flow Cell
材料
- Flow Cell Wash Kit (Q-EXP-WSH004)
装置
- P1000 ピペット及びチップ
- P20 ピペットとチップ
- アイスバケツ(氷入り)
Preparation to run the washing procedure
- This protocol assumes that the flow cell has already had a library run on it.
- The aim is to remove the initial library and prepare the flow cell for the loading of a subsequent library.
- The Flow Cell Wash Kit contains all solutions required for removal of the initial library.
- After washing the flow cell, you can load a new library or store the flow cell at 4°C.
If you need to wash the flow cell in the middle of a run, cancel the assay in the sequencing software.
Under the flow cell position, click View run. 
Then click Cancel run. 
Place the tube of Wash Mix (WMX) on ice. Do not vortex the tube.
Thaw one tube of Wash Diluent (DIL) at room temperature.
Mix the contents of Wash Diluent (DIL) thoroughly by vortexing, then spin down briefly and place on ice.
In a fresh 1.5 ml Eppendorf DNA LoBind tube, prepare the following Flow Cell Wash Mix:
| Reagent | Volume per flow cell |
|---|---|
| Wash Mix (WMX) | 2 μl |
| Wash Diluent (DIL) | 398 μl |
| Total | 400 μl |
Mix well by pipetting, and place on ice. Do not vortex the tube.
Slide the flow cell priming port cover clockwise to open.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
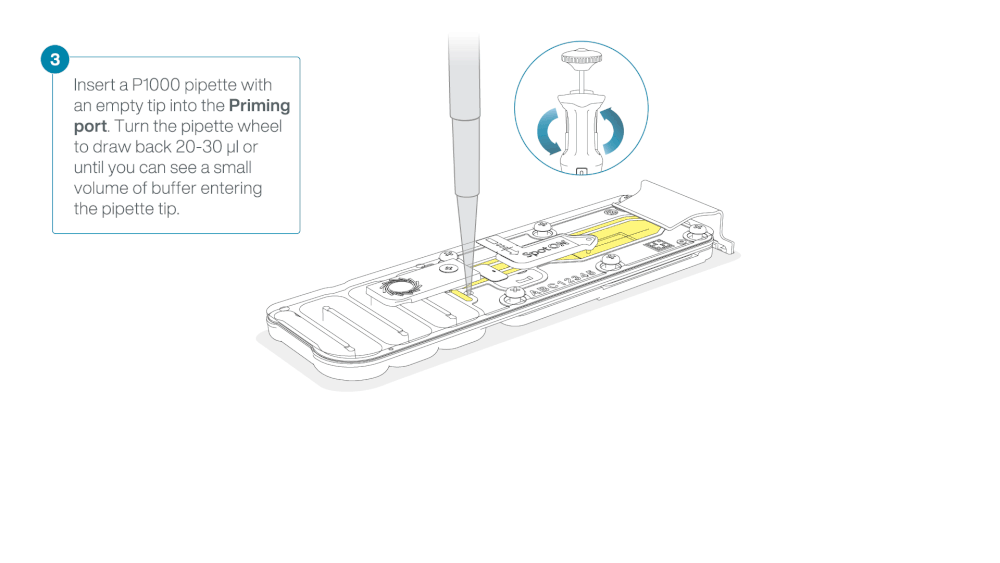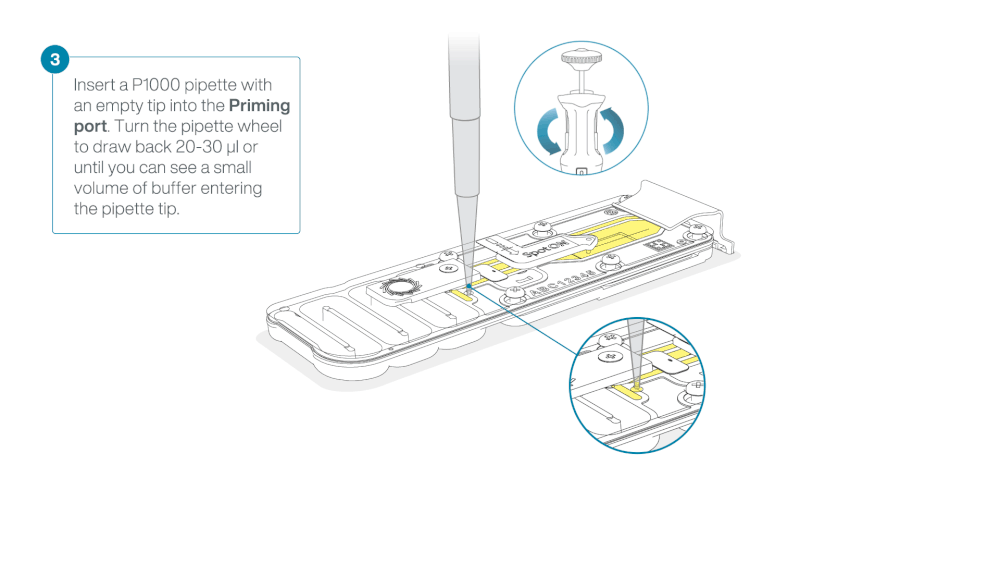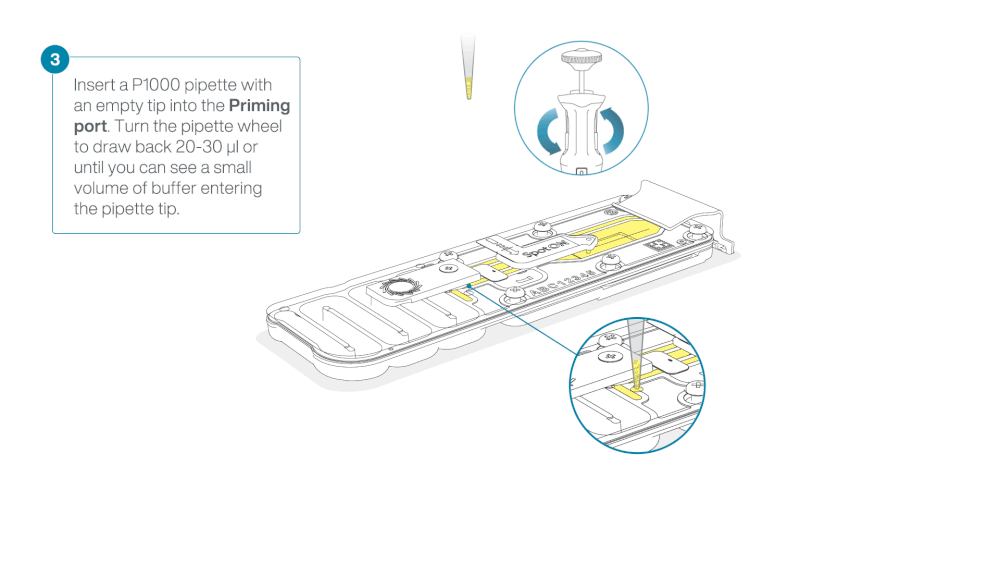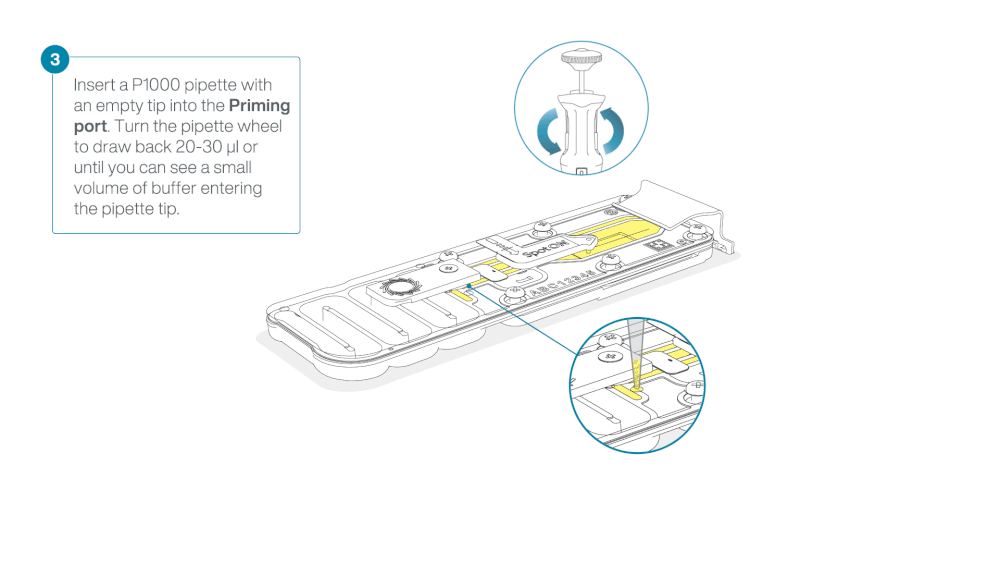
Check for air between the flow cell priming port and the sensor array. If necessary, using Use a P1000 to draw back a small volume to remove any air:
- Set a P1000 pipette to 200 µl.
- Insert the tip into the flow cell priming port.
- Turn the wheel until the dial shows 220-230 µl, or until you can see a small volume of buffer/liquid entering the pipette tip.
- Visually check that there is continuous buffer from the flow cell priming port across the sensor array.
Remove your prepared Flow Cell Wash Mix from ice and allow to come to room temperature immediately prior to loading it onto the flow cell .
Slowly load 200 µl of the prepared flow cell wash mix into the priming port, as follows:
- Using a P1000 pipette, take 200 µl of the flow cell wash mix.
- Insert the pipette tip into the priming port, ensuring there are no bubbles in the tip.
- Push down the plunger very slowly (for ~10 seconds), leaving a small volume of buffer in the pipette tip.
- Set a timer for a five minute incubation.
Return the remaining Flow Cell Wash Mix to ice.
Once the five minute incubation is complete, carefully load the remaining 200 µl of the prepared flow cell wash mix into the priming port, as follows:
- Using a P1000 pipette, take the remaining 200 µl of the flow cell wash mix.
- Insert the pipette tip into the priming port, ensuring there are no bubbles in the tip.
- Push down the plunger very slowly (for ~10 seconds), leaving a small volume of buffer in the pipette tip.
Close the flow cell priming port and set a timer for a 60-minute incubation.
It is vital that the flow cell priming port and SpotON sample port are closed before removing the waste buffer to prevent air from being drawn across the sensor array area, which would lead to a significant loss of sequencing channels.
Remove the waste buffer as follows:
- Close the priming port and SpotON sample port cover, as indicated in the figure below.
- Insert a P1000 pipette into waste port 1 and remove the waste buffer.
Note: As both the priming port and SpotON sample port are closed, no fluid should leave the sensor array area.

Thaw one tube of Storage Buffer (S) at room temperature.
Mix contents thoroughly by pipetting and spin down briefly.
Rotate the flow cell priming port cover clockwise so that the priming port is visible.
Check for air between the flow cell priming port and the sensor array. If necessary, using Use a P1000 to draw back a small volume to remove any air:
- Set a P1000 pipette to 200 µl.
- Insert the tip into the flow cell priming port.
- Turn the wheel until the dial shows 220-230 µl, or until you can see a small volume of buffer/liquid entering the pipette tip.
- Visually check that there is continuous buffer from the flow cell priming port across the sensor array.

Slowly add 500 μl of Storage Buffer (S) through the flow cell priming port, as follows:
- Using a P1000 pipette, take 500 µl of the Storage Buffer.
- Insert the pipette tip into the priming port, ensuring there are no bubbles in the tip.
- Push down the plunger very slowly (for ~20 seconds), leaving a small volume of buffer in the pipette tip.
Close the priming port.
It is vital that the flow cell priming port and SpotON sample port are closed before removing the waste buffer to prevent air from being drawn across the sensor array area, which would lead to a significant loss of sequencing channels.
Remove all fluid from the waste channel through waste port 1 using a P1000 pipette.
As both the flow cell priming port and SpotON sample port are closed, no fluid should leave the sensor array area.
The flow cell can now either be re-used or stored at 4-8°C in the blister pack provided.
Note: If you store the flow cell at 4-8°C, when you come to reuse it, remove the flow cell from storage and allow it to warm to room temperature for ~5 minutes.
If you are re-using the flow cell straight away, run a flow cell check.
Click on View flow cell.
Under "Flow cell maintenance", click on Run flow cell check.
After the flow cell has passed the check, prime and load your flow cell for the next assay.







