MinION and flow cells (HWTD_5000_v1_revP_07March2025)
TechnicalDocument
MinION and flow cells V HWTD_5000_v1_revP_07March2025
For Research Use Only
FOR RESEARCH USE ONLY
Contents
Introduction to the MinION Mk1B and Mk1C
MinION safety and regulatory information
- 4. Prerequisites for using the MinION Mk1B and Mk1C
- 5. Safety information for using the MinION Mk1B and Mk1C
- 6. MinION Mk1B Regulatory information
Flow cell components
Thermal control of MinION Mk1B and Mk1C
Descripción general
For Research Use Only
1. The MinION Mk1B and Mk1C
The MinION Mk1B
The MinION Mk1B instrument is an electronic device that provides the interface between the user’s PC and the nanopore sensor. It also provides power to the application-specific integrated circuit (ASIC), performs temperature control, shields the sensor from electronic noise, and transfers data to the PC. The device measures 105 mm x 33 mm x 23 mm.
The MinION Mk1B has a sensor and control program to maintain the temperature during a sequencing run and dissipate the heat generated from the ASIC. There is no active heating element in the device; heat from the ASIC and control of the fan speed are sufficient to allow the MinION Mk1B to maintain a steady temperature of 37° C in environments ranging from 18° C to 24° C.
The metal case of the MinION Mk1B is electrically grounded to the printed circuit board and protects the sensor from electric fields. Continuity between PCB, instrument enclosure and lid is provided by magnetic contacts and a conductive hinge assembly. A magnetic shield is also present to further reduce noise in the signal.
Power and data transfer is achieved through a single USB 3.0 port. This provides all power required by the instrument and ASIC (5 V @ 900 mA max). Data transfer rates on USB 3.0 are sufficient to handle all 512 recording channels outputting raw data at 33 kHz.
The MinION Mk1B is shipped together with a USB cable and a Configuration Test Cell.

The MinION Mk1B is adaptable for DNA, RNA sequencing, protein sensing and other nanopore sensing techniques. The MinION Mk1B is operated using the MinKNOW™ software, which also performs basecalling. Other basecalling and analysis options are available in Oxford Nanopore's range of software.
The MinION Mk1C
The MinION Mk1C combines the real-time, rapid, portable sequencing of a MinION Mk1B with a Graphical Processing Unit (GPU) and a high-resolution screen. The device applies the technology of the MinION Mk1B with the basecalling power of the MinIT and the additional features of smartphone, such as a cellular modem and touch screen.

MinKNOW is the control software for Oxford Nanopore Technologies sequencing platforms and it comes installed on the MinION Mk1C, without the need of a laptop.
2. MinION Configuration Test Cell
Configuration test cell (CTC)
The hardware check is the process of testing that communication between the MinION Mk1B and Mk1C device and the control software on the host computer is operational prior to experimental work being performed. This is carried out in the absence of any chemistry and uses a specific flow cell known as the Configuration Test Cell (CTC).

3. Data connection
USB cable and port
LEDs
To power on the MinION Mk1C
Use the AC/DC adapter included with the device to power the MinION Mk1C.
Please see the Power section of the MinION Mk1C protocol.
4. Prerequisites for using the MinION Mk1B and Mk1C
Prerequisites for using the MinION Mk1B and Mk1C
In order to operate the systems the way they are intended, the following prerequisites must be fulfilled:
- You should have a general understanding of how to use the personal computer and operating system intended to operate the device
- You must read and understand the safety instructions
- The instrument and software should be installed, configured and tested according to the Configuration guide
- You should understand the concepts of nanopore sensing
5. Safety information for using the MinION Mk1B and Mk1C
Intended use of the MinION Mk1B and Mk1C
Oxford Nanopore Technologies® MinION device is an electronic analysis system for use in scientific research. The core technology is built around a nanopore that is able to detect single molecule events including nucleic acids (DNA/RNA), proteins and small molecules.
It is for research use only and not for in vitro diagnostic or therapeutic use.
The purpose of the safety information
The safety information provides you with the details needed to install and use the system safely.
MinION Mk1B electrical output values
| Supply voltage | 5 V |
| Operating current | 800 mA for 10 k 900 mA for 33 k 1 A maximum |
| Maximum power | 5 W |
MinION Mk1C electrical output values
| Power supply | 6.3-19.6 VDC |
| Max rated current | 10 A |
| Max rated power | 60 W |
Safety notices

WARNING: indicates a hazardous situation which, if not avoided, could result in death or serious injury. It is important not to proceed until all stated conditions are met and clearly understood.

CAUTION: indicates a hazardous situation which, if not avoided, could result in minor or moderate injury. It is important not to proceed until all stated conditions are met and clearly understood.

ADVISORY: indicates instructions that must be followed to avoid damage to the product or other equipment.
The safety notices below are intended to supplement, not supersede, the normal safety requirements prevailing in the user’s country.
Material Safety Data Sheets (MSDSs)
The latest versions of the Material Safety Data Sheets are available in the Nanopore Store. Each product has a Safety and Legal tab, and the MSDSs are saved here and can be downloaded as required. For further assistance, please contact support@nanoporetech.com .

Read and understand the MSDSs before handling, working with or storing the chemicals being used within the Oxford Nanopore Technologies® sequencing device.
Minimise contact with the chemicals by wearing protective clothing, safety glasses and gloves. The MSDS will carry specific requirements.
Minimise inhalation of chemicals by using appropriate and adequate ventilation. The MSDS will carry any specific requirements.
Continuously check for any spills or leakages. If a spill or leak occurs, follow the cleanup guidelines provided on the MSDS.
All components of the MinION devices system should be handled, stored and disposed of in accordance with local, state/provincial or national laws and regulations.
General precautions

When handling toxic, radioactive or pathogenic samples as defined by the WHO Laboratory Biosafety Manual, observe the safety regulations of the specific local in question.

Do not use the MinION device if it has suffered any damage, e.g. to power cables, data transfer cables, power supplies, or flow cells.
Personal protection

Specimens and reagents containing materials from humans should be treated as potentially infectious. Use safe laboratory procedures as outlined in publications such as Biosafety in Microbiological and Biomedical Laboratories (http://www.cdc.gov/biosafety/publications/bmbl5/index.htm).

The operator has to take all necessary actions to avoid spreading hazardous biological agents in the vicinity of the system. The facility should comply with the national code of practice for biosafety.

Samples being loaded into the flow cell should be used, stored and disposed of according to the required safety regulations and laws. Consult the responsible body for safety in your lab for local regulations.

Samples containing infectious agents should be handled with the greatest of care and in accordance with the required safety regulations and laws.

It is good laboratory practice to always wear safety glasses, gloves (two pairs if working with infectious agents) and a lab coat. There may be other locally advised items which add to this recommendation.
Use of the MinION Mk1B and Mk1C, flow cells and reagents

When handling toxic, radioactive or pathogenic samples as defined by the WHO Laboratory Biosafety Manual, observe the specific local regulations.

Loading excess buffer, sample or de-ioinised water to the Flow Cell will cause an overflow of the waste compartment. Absorbent material should be used to capture sample and buffer which will come out through the waste port. All material should be disposed of in line with local regulations for biological waste.

In the unlikely event that the sequencing device is found to be hot during use, disconnect the device from the host computer and refer to troubleshooting guidelines.
Maintenance

Repairs must only be performed by Oxford Nanopore Technologies, and no components should be replaced. Contact support@nanoporetech.com in the event of damage to the sequencing device or flow cells.

Before using cleaning or decontamination methods other than those stipulated by the manufacturer, contact the manufacturer to ensure that the intended method will not damage the sequencing device and/or flow cell.

When returning sequencing devices and/or flow cells, ensure that they are fully decontaminated and do not present any health risk to our staff.
Disposal and recycling instructions

Used plasticware, such as reagents, tubes and pipette tips, must be collected and disposed of properly in accordance with local safety regulations and laboratory procedures.

The flow cell buffer, wash kit buffers, and library preparation kit buffers must not be mixed and must be kept away from strong acids and alkalis.

The flow cell buffer, wash kit buffers, and library preparation kit buffers must be disposed of according to the local regulations. They must not be disposed of down a sink.

The Terms and Conditions for the use of sequencing devices stipulate that any flow cells that have been used with or otherwise been in contact with materials of Biohazard Level 3 or higher (“ Contaminated Flow Cells ”) must not be returned. Proof of legal and appropriate destruction of any contaminated Flow Cells will be required.

The sequencing device shall be decontaminated before decommissioning, and all local regulations for electronic and electrical waste shall be followed with regard to disposal of the components if they are not being returned to Oxford Nanopore Technologies.

The flow cell shall be disposed of as hazardous biological waste, and all local regulation for such waste shall be followed if they are not being returned to Oxford Nanopore Technologies.
Emergency procedures
In case of emergency, switch the computer off at the power switch and unplug the USB 3.0 cable from the computer.
6. MinION Mk1B Regulatory information
Declaration of conformity
International standards
The MinION Mk1B is certified to the following international standards:
| Certification | Country |
|---|---|
| MET; UL61010/CSA-C22.2 No. 61010, third Edition: Electrical Equipment for Measurement, Control and Laboratory Use, Revision: May 11, 2012 | USA and Canada |
| RCM compliance | Australia and New Zealand |
7. SpotON™ Flow Cells
The SpotON™ Flow Cells
The Flow Cells contain the proprietary sensor array, Application-Specific Integrated Circuit (ASIC) and nanopores that are needed to perform a complete single-molecule sensing experiment.
Two flow cells are included in the Starter Pack, and they can also be purchased separately.

Flow cell packaging
The flow cells are shipped in a sealed pouch to maintain the environment for the flow cell chemistry.

Flow cells are shipped at a constant temperature to avoid temperature cycling, and for long term storage we recommend that they are kept in an environment not prone to temperature fluctuations. You should store your flow cells:
- For 12 weeks chilled at 2-8º C
Note: Do not freeze your flow cells, this will lead to component damage and irreparable pore loss.
Returns packaging
The flow cells are supplied with the packaging materials for returns to Oxford Nanopore.
The returns packaging includes:
- Flow cell container seal
- Plastic shipping container (protects the flow cell during shipments)
- Flow cell pouch (keep when unpacking the flow cell)
- External envelope
The envelopes and seals can be found in the flow cell packaging.
8. Appearance
Flow cell appearance
The flow cell is a disposable element of the sequencing platform that provides the fluidic interface between the nanopores and the electrodes, allowing the user’s sample to be analysed.
The flow cell is composed of: a moulded plastic fluidic chamber and retainer, a fluidic gasket, and the sensor chip containing a printed circuit board (PCB) and an application specific integrated circuit (ASIC).
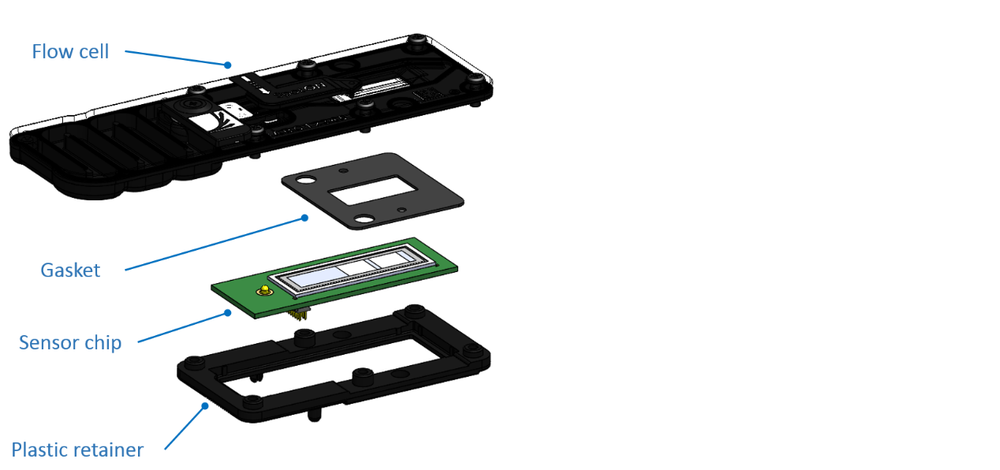
The flow cell dimensions are 92.4 mm x 28.0 mm x 5.8 mm; on the upper side all the ports and sensor chip are visible. The flow cell clips into the MinION Mk1B, Mk1C or GridION device.
The fluidics of the flow cell are described below. The sensor array is where the sequencing chemistry happens. The array is made up of 512 sensing wells, each designed to collect sequencing data through a single nanopore.

The flow cell itself is designed with a number of distinct areas. These areas include: the priming port, SpotON port, sensor array, waste channel, and two waste ports. Sample can be added directly onto the nanopore array via the SpotON port. The volume of the main chamber area over the sensor array is 100 μl, while the waste can hold 2 ml.
Flow cell valves The path of the liquid through the flow cell is regulated by two valves: one immediately downstream of the priming port, and the other between the end of the array and the waste chamber (valves shown in the diagram below in red). The flow cells are shipped with the priming port covered and both valves closed.

Waste chamber The waste chamber has a total capacity of 2 ml and is accessible when the valve at the end of the array is open. Waste port 2 allows excess sample to be removed with a pipette if, for instance, the flow cell is topped up mid-run.

Storage buffer
The flow cell has three compartments: the common electrode reservoir which is separated from the bulk by a diffusion barrier, the bulk which is separated from the individual wells by a membrane, and the individual wells. The flow cells are shipped with the storage buffer (yellow) in all three compartments to maintain osmotic balance. The storage buffer contains salt and the standard redox couple that enables current to run through the nanopore. The storage buffer also contains a QC DNA molecule that gives a distinct signal which helps to identify functional pores during Platform QC.

Before library loading, the flow cell is flushed with Priming mix (a mixture of RBF and nuclease-free water, shown as blue), which displaces the storage buffer from the bulk compartment.

9. Flow cell chip
ASIC
The ASIC is a high-density array of low-noise amplifier circuits that is used to measure and provide a digital readout of the current flow in the electrochemical circuit between the individual well and shared common electrode. In addition, the ASIC receives commands from the host software system to provide control functionality for the sensor array, including: acquisition frequency, signal filtering, sensor current range, multiplex input selection, electrode bias potential generation, and deselection of sensor inputs with broken membranes to avoid saturation of the measurement circuits.
The flow cell ASIC is designed to operate across a wide range of conditions depending on the application. Typical operating cases are acquisition frequency of 2 to 20 kHz while measuring 10s to 1000s of pico-amperes. The applied electrode bias potential can be controlled using scripts in MinKNOW with a range of ± 1 V.
The ASIC also has the ability to use the applied potential to unblock any of the channels that are not sequencing. A reversal of the potential can “flick” any stray DNA or contaminants out of the pore on a per channel basis and reset the channel to an “open pore” state to allow the next strand to be sequenced. This ability is further utilised in “Read Until...” schemes, where the MinION Mk1B and Mk1C can be programmed to responds to a sequence or signature, choosing to either keep or reject the DNA stand in the nanopore on an individual basis, maximising the time to results and allowing the user to change the priority of sequencing during a run.

Channel layout
The MinION Mk1B and Mk1C/GridION flow cell currently has 2048 active well electrodes organised hexagonally on the surface of the array. The active wells are arranged in two blocks of 32x32 with a set of four inactive wells separating them. There are also inactive wells present at the edges of the array where the gasket sits.
The channel map is shown below, viewing the sensor chip from the top with the fluid inlet (or common connection pin) to the left of the sensor.

Layout:
- The current hexagonal packing is a result of the horizontal offset of alternative rows and the original chip design evolving from a square array
- There is a section of four columns in the middle where there are wells on the surface of the chip, but these wells are not connected to the ASIC (black hexagons)
- There are also some redundant wells around the edges of the sensor (black hexagons)
Basic numbers:
- 2048 sensor wells available (64 x 32)
- 512 measurement channels, 4x Multiplexing (MUX), see the MUX scan below.
Features:
- Channels 1-64 occur along the top rows of the chip (the other low channel numbers are at the bottom)
- Channels order down the chip: 1-64, 449-512, 385-448, 321-384, 257-320, 193-256, 129-192, 65-128
- MUXs run from left to right in the order: 3, 4, 1, 2, 2, 1, 4, 3
Relationship between nanopore sensors and ASIC channels
The ASIC is capable of recording through all channels simultaneously, with the 2048 active well electrodes organised in groups of four. The choice of a single well for each of the recording channels is referred to as multiplexing. Multiplexing the nanopore array is used to improve the yield of channels containing a single pore and the sequencing output of the consumable. Is it common to see data provided with ‘channel’ and ‘MUX’ information. Most sequencing scripts perform a ‘MUX selection’ where the best 512 wells are chosen as the first group. Later groups of wells can be switched to during a sequencing run to provide more output.

Sensor array
The sensor area of the flow cell is comprised of many individual sensor wells, each designed to hold a single nanopore. These sensor wells have a micro patterned structure, created using photolithographic techniques, sitting on a silicon substrate. The well provides the geometry for forming a membrane so that a single nanopore can be inserted. The well is approximately 90 μm deep with a platinum electrode at the base of the well. This electrode is then connected to the PCB through vias in the silicon layer. On the top of the wells are pillars of photoresist that are patterned to control the movement of a hydrophobic pretreatment. The wells are also patterned with additional features to control the pretreatment distribution.
 SEM image of a single sensor well (left) and the hexagonal array of wells (right).
SEM image of a single sensor well (left) and the hexagonal array of wells (right).
Membranes
For each of the wells, a membrane is formed over the array so that the ionic fluid underneath the membrane is trapped. The membrane provides an insulating layer, so that once a nanopore is inserted, communication between the electrodes is dominated by the signal through that pore. Oxford Nanopore uses a proprietary amphiphilic polymer and a synthetic pretreatment oil to form the membrane. The use of this polymer gives the flow cell membranes increased robustness to physical disruption, chemicals, and biological materials.
Electrodes
Electrodes are used to create a circuit with the membrane and the buffer, present in the sensor array, so that electrophysiological measurements can be made as the ion flow changes through the pores.
10. Flow cell checks and MUX scans
Factors contributing to the nanopore signal
The nanopore and molecules interacting with the nanopore
E.g. the motor protein.
Applied voltage
- Set voltage, defined in the protocol scripts in MinKNOW
- The resistance of the electrochemical path between the common electrode and the well electrode
- The electrochemistry at the electrodes
Temperature
Higher temperatures increase the mobility of charged species and thereby increase the current. A range of set temperatures is used for various steps of the sequencing run:

Buffer
The storage and running buffers contain different concentrations of charged species, and will affect the pores and DNA translocation differently.
Platform QC
The choice of which well is used is made via the multiplexer (MUX), a switch which allows the selection of the best channels for sequencing. For a summary of the MUX layout, please see the channel layout description above. The Platform QC is a scan of each MUX in turn to see if a pore is present, with the aim of determining the total number of functional pores present in a chip. Platform QC must be run on a new flow cell within ten days of receipt.
The Platform QC script runs through each MUX in turn, first applying a triangle wave to assess the membrane. Then 180 mV are applied for 30 s, and different metrics contained in the signal, such as current levels, noise measurements and events that can help to classify the signal, are recorded. The pores are classified as 'single pore', 'multiple pores', 'saturated', and 'zero current'. To class a single pore, the software searches for an open pore current level within a given range, combined with the distinct signal of the QC DNA strand that is present in the flow cell storage buffer. The MinKNOW GUI then displays the total number of single pores.
The MUX scan
At the beginning of the sequencing run, when a library has been loaded, an additional MUX scan looks at the performance of the nanopores and groups them into collections of 512 to determine which one will provide the best signal and therefore the best sequence during the time of the run. In a similar fashion to the Platform QC, a 180 mV voltage is applied to each MUX in turn. If there are library DNA strands in any of the pores, these are now used to classify channels. Channels are prioritised for sequencing based on 'strand' first, then 'pore', then 'unavailable' pores.
- strand - a DNA strand translocating through a single pore
- pore - an open pore without DNA
- unavailable - signals that the software is not confident about
The MUX scan report shows the number of active pores, defined as channels in strand and open pore. MinKNOW then groups the best 512 channels for sequencing plus 2nd and 3rd choices that can be utilised during a sequencing run. This is the groups 1, 2, and 3 reported on the GUI. Sequencing is begun from group 1.
Voltage drift during a sequencing run
Once the MinKNOW script proceeds to sequencing, the system goes through a cycle of voltage changes. The current scripts start with an applied voltage of -180 mV, which is optimal for basecalling with R9.4.1 chemistry. However in the process of a run, the voltage drifts due to the depletion of the redox chemistry in the bulk solution.
In order to keep the voltage constant, MinKNOW enables "Dynamic Voltage Control", which constantly monitors the raw signal from the device, and adjusts the applied voltage when required. Dynamic Voltage Control (in black) maintains a considerably tighter grouping of current levels, which is more stable over the course of a run than that previously achieved by the Static Voltage Control used in earlier versions of MinKNOW:

11. Components for temperature control
Local environment
The MinION Mk1B and Mk1C are able to maintain a heatsink temperature of 37°C on a typical lab bench when the local ambient conditions are between 19°C and 24°C. However, there are a number of external factors which can disrupt the local conditions and which need to be taken into account, for example warm air expelled from laptops, or cool air from a fan or air conditioning system increasing airflow around the MinION Mk1B and Mk1C.
The MinION Mk1B and Mk1C takes approximately 10 minutes to get to temperature. If the heatsink temperature is not running at 37°C, there are a number of simple measures that can bring the MinION Mk1B and Mk1C to the correct running temperature.
MinION Mk1B and Mk1C temperature
During an experiment, the heatsink temperatures is shown to the user by the MinKNOW software. Once the heatsink reaches the correct temperature, it remains at a constant throughout the experiment.
Fan control
To maintain the operating temperature range, the MinION Mk1B and Mk1C houses a fan. It is located under the heat pad recess and helps to dissipate heat away from the ASIC. It will switch on and off as required. While it is running, a fan noise may be audible.











