Ligation sequencing gDNA - native barcoding (Q-SQK-LSK109 with Q-EXP-NBD104 and Q-EXP-NBD114) (Q_NBD_revB_26Sep2024)
GridION: Protocol
V Q_NBD_revB_26Sep2024
FOR RESEARCH USE ONLY
Contents
Introduction to the protocol
Library preparation
- 4. DNA repair and end-prep
- 5. Native barcode ligation
- 6. Adapter ligation and clean-up
- 7. Priming and loading the MinION Flow Cell
Sequencing and data analysis
1. Overview of the protocol
Native Barcoding Expansion 1-12 and 13-24 features
These kits are recommended for users who:
- wish to multiplex samples to reduce price per sample
- need a PCR-free method of multiplexing to preserve additional information such as base modifications
- want to optimise their sequencing experiment for throughput
- require control over read length
- are interested in utilising upstream processes such as size selection or whole genome amplification
Introduction to the Ligation Sequencing Kit
The Ligation Sequencing Kit is designed to prepare genomic, amplicon, and cDNA, with or without barcoding, for sequencing on Oxford Nanopore devices to produce 1D reads. This kit can be used to prepare libraries from whole genome amplified DNA, starting with as little as 10 pg genomic DNA.
The kit contains an adapter, which must be ligated onto end-repaired and A-tailed fragments. This adapter is loaded with the motor protein that translocates DNA through the nanopore. The workflow for nanopore sequencing consists of steps for template preparation, and then steps required for adapter ligation.
This protocol describes how to carry out native barcoding of genomic DNA using the Native Barcoding Expansion 1-12 (Q-EXP-NBD104) and 13-24 (Q-EXP-NBD114), in conjunction with the Ligation Sequencing Kit (Q-SQK-LSK109). There are 24 unique barcodes if using both expansion kits, allowing the user to pool up to 24 different samples in one sequencing experiment. It is highly recommended that a Lambda control experiment is completed first to become familiar with the technology.
Steps in the sequencing workflow
Prepare for your experiment
You will need to:
- Extract your DNA and check its length, quantity, and purity. The quality checks performed during the protocol are essential in ensuring experimental success.
- Ensure you have your sequencing kit, the correct equipment and third-party reagents
Prepare your library
You will need to:
- Repair the nicks in the DNA and prepare the DNA ends for adapter attachment
- Ligate Native barcodes supplied in the kit to the DNA ends
- Ligate sequencing adapters, supplied in the kit, to the DNA ends
- Prime the flow cell and load your DNA library into the flow cell
Sequencing
You will need to:
- Start an assay using the sequencing software, which will collect raw data from the device and convert it into basecalled reads. There is also an option to demultiplex reads into barcode-specific folders.
We do not recommend mixing barcoded libraries with non-barcoded libraries prior to sequencing.
Compatibility of this protocol
This protocol should only be used in combination with:
- Ligation Sequencing Kit (Q-SQK-LSK109)
- Native Barcoding Expansions 1-12 (Q-EXP-NBD104) and 13-24 (Q-EXP-NBD114)
- Q-FLO-MIN106D flow cells
- Control Expansion (Q-EXP-CTL001)
2. Equipment and consumables
Material
- 1 µg (or 100-200 fmol) high molecular weight genomic DNA for every sample to be barcoded
- o 100+ ng de ADN genómico de alto peso molecular (si se fragmenta el ADN).
- Native Barcoding Expansion 1-12 (Q-EXP-NBD104) and 13-24 (Q-EXP-NBD114) if multiplexing more than 12 samples
- Ligation Sequencing Kit (Q-SQK-LSK109)
- Flow Cell Priming Kit (Q-EXP-FLP002)
- SpotON Flow Cell (Q-FLO-MIN106D)
Instrumental
- Mezclador Hula (mezclador giratorio suave)
- Gradilla magnética, adecuada para tubos Eppendorf de 1,5 ml
- Microcentrífuga
- Mezclador vórtex
- Termociclador
- Pipeta y puntas P1000
- Pipeta y puntas P200
- Pipeta y puntas P100
- Pipeta y puntas P20
- Pipeta y puntas P10
- Pipeta y puntas P2
- Cubeta con hielo
- Temporizador
Equipo opcional
- Bioanalizador Agilent (o equivalente)
- Fluorímetro Qubit (o equivalente)
- Centrifugadora Eppendorf 5424 (o equivalente)
For this protocol, you will need 1 µg (or 100-200 fmol) high molecular weight genomic DNA.
Although 1 µg (or 100-200 fmol) gDNA is recommended, users can start with lower input quantities (down to 100 ng) if performing DNA fragmentation to increase the number of DNA molecules in the sample, or if amplifying the sample by PCR.
Input DNA
How to QC your input DNA
It is important that the input DNA meets the quantity and quality requirements. Using too little or too much DNA, or DNA of poor quality (e.g. highly fragmented or containing RNA or chemical contaminants) can affect your library preparation.
For instructions on how to perform quality control of your DNA sample, please read the Q system input DNA QC protocol.
NEBNext® Companion Module for Oxford Nanopore Technologies® Ligation Sequencing
For customers new to nanopore sequencing, we recommend buying the NEBNext® Companion Module for Oxford Nanopore Technologies® Ligation Sequencing (catalogue number E7180S or E7180L), which contains all the NEB reagents needed for use with the Ligation Sequencing Kit.
Please note, for our amplicon protocols, NEBNext FFPE DNA Repair Mix and NEBNext FFPE DNA Repair Buffer are not required.
Native Barcoding Expansion 1-12 (Q-EXP-NBD104) and 13-24 Q-(EXP-NBD114) contents
Q-EXP-NBD104 kit contents
Q-EXP-NBD114 kit contents
Ligation Sequencing Kit (Q-SQK-LSK109) contents
Flow Cell Priming Kit (Q-EXP-FLP002) contents

Native barcode sequences
The native barcode sequences are the reverse complement of the corresponding barcode sequence in other kits.
Native Barcoding Expansion 1-12 and 13-24 (Q-EXP-NBD104 and Q-EXP-NBD114)
| Component | Forward sequence | Reverse sequence |
|---|---|---|
| NB01 | CACAAAGACACCGACAACTTTCTT | AAGAAAGTTGTCGGTGTCTTTGTG |
| NB02 | ACAGACGACTACAAACGGAATCGA | TCGATTCCGTTTGTAGTCGTCTGT |
| NB03 | CCTGGTAACTGGGACACAAGACTC | GAGTCTTGTGTCCCAGTTACCAGG |
| NB04 | TAGGGAAACACGATAGAATCCGAA | TTCGGATTCTATCGTGTTTCCCTA |
| NB05 | AAGGTTACACAAACCCTGGACAAG | CTTGTCCAGGGTTTGTGTAACCTT |
| NB06 | GACTACTTTCTGCCTTTGCGAGAA | TTCTCGCAAAGGCAGAAAGTAGTC |
| NB07 | AAGGATTCATTCCCACGGTAACAC | GTGTTACCGTGGGAATGAATCCTT |
| NB08 | ACGTAACTTGGTTTGTTCCCTGAA | TTCAGGGAACAAACCAAGTTACGT |
| NB09 | AACCAAGACTCGCTGTGCCTAGTT | AACTAGGCACAGCGAGTCTTGGTT |
| NB10 | GAGAGGACAAAGGTTTCAACGCTT | AAGCGTTGAAACCTTTGTCCTCTC |
| NB11 | TCCATTCCCTCCGATAGATGAAAC | GTTTCATCTATCGGAGGGAATGGA |
| NB12 | TCCGATTCTGCTTCTTTCTACCTG | CAGGTAGAAAGAAGCAGAATCGGA |
| NB13 | AGAACGACTTCCATACTCGTGTGA | TCACACGAGTATGGAAGTCGTTCT |
| NB14 | AACGAGTCTCTTGGGACCCATAGA | TCTATGGGTCCCAAGAGACTCGTT |
| NB15 | AGGTCTACCTCGCTAACACCACTG | CAGTGGTGTTAGCGAGGTAGACCT |
| NB16 | CGTCAACTGACAGTGGTTCGTACT | AGTACGAACCACTGTCAGTTGACG |
| NB17 | ACCCTCCAGGAAAGTACCTCTGAT | ATCAGAGGTACTTTCCTGGAGGGT |
| NB18 | CCAAACCCAACAACCTAGATAGGC | GCCTATCTAGGTTGTTGGGTTTGG |
| NB19 | GTTCCTCGTGCAGTGTCAAGAGAT | ATCTCTTGACACTGCACGAGGAAC |
| NB20 | TTGCGTCCTGTTACGAGAACTCAT | ATGAGTTCTCGTAACAGGACGCAA |
| NB21 | GAGCCTCTCATTGTCCGTTCTCTA | TAGAGAACGGACAATGAGAGGCTC |
| NB22 | ACCACTGCCATGTATCAAAGTACG | CGTACTTTGATACATGGCAGTGGT |
| NB23 | CTTACTACCCAGTGAACCTCCTCG | CGAGGAGGTTCACTGGGTAGTAAG |
| NB24 | GCATAGTTCTGCATGATGGGTTAG | CTAACCCATCATGCAGAACTATGC |
3. Computer requirements and software
GridION IT requirements
The GridION device contains all the hardware required to control up to five sequencing experiments simultaneously and acquire the data. The device is further enhanced with high performance GPU technology for real-time basecalling. Read more in the GridION Q IT Requirements document in the Q system channel.
The sequencing software
The sequencing software controls the GridION, collects sequencing data in real-time and processes it into basecalls. The software can also demultiplex reads by barcode, and basecall/demultiplex data after a sequencing run has completed. You will be using the sequencing software for every assay you run.
For instructions on how to run the sequencing software on the GridION, please refer to the Q-Line sequencing software user guide.
4. DNA repair and end-prep
Material
- gDNA in 47 µl nuclease-free water
Consumibles
- Tubos de PCR de pared fina (0,2 ml)
- Agua sin nucleasas (p. ej., ThermoFisher AM9937)
- NEBNext FFPE DNA Repair Mix (NEB M6630)
- Módulo NEBNext® Ultra™ II End Repair/dA-Tailing (NEB, E7546)
- Microesferas Agencourt AMPure XP (Beckman Coulter™, A63881)
- Etanol al 80 % recién preparado con agua sin nucleasas
- Tubos Eppendorf DNA LoBind de 1,5 ml
Instrumental
- Pipeta y puntas P1000
- Pipeta y puntas P100
- Pipeta y puntas P10
- Termociclador
- Microcentrífuga
- Mezclador Hula (mezclador giratorio suave)
- Gradilla magnética
- Cubeta con hielo
Program a thermal cycler to run at 20°C for 5 minutes and 65°C for 5 mins.
Prepare the NEBNext FFPE DNA Repair Mix and NEBNext Ultra II End Repair / dA-tailing Module reagents in accordance with manufacturer’s instructions, and place on ice.
For optimal performance, NEB recommend the following:
Thaw all reagents on ice.
Flick and/or invert the reagent tubes to ensure they are well mixed.
Note: Do not vortex the FFPE DNA Repair Mix or Ultra II End Prep Enzyme Mix.Always spin down tubes before opening for the first time each day.
The Ultra II End Prep Buffer and FFPE DNA Repair Buffer may have a little precipitate. Allow the mixture to come to room temperature and pipette the buffer up and down several times to break up the precipitate, followed by vortexing the tube for 30 seconds to solubilise any precipitate.
Note: It is important the buffers are mixed well by vortexing.The FFPE DNA Repair Buffer may have a yellow tinge and is fine to use if yellow.
Prepare the DNA in nuclease-free water.
Transfer 1 μg (or 100-200 fmol) genomic DNA into a 1.5 ml Eppendorf DNA LoBind tube Adjust the volume to 48 μl with nuclease-free water Mix thoroughly by flicking the tube to avoid unwanted shearing Spin down briefly in a microfuge
In a 0.2 ml thin-walled PCR tube, mix the following:
| Reagent | Volume |
|---|---|
| DNA | 48 µl |
| NEBNext FFPE DNA Repair Buffer | 3.5 µl |
| Ultra II End-prep reaction buffer | 3.5 µl |
| Ultra II End-prep enzyme mix | 3 µl |
| NEBNext FFPE DNA Repair Mix | 2 µl |
| Total | 60 µl |
Mix well by pipetting using wide-bore pipette tips. Alternatively, if you are concerned about preserving the integrity of very long DNA fragments, mix gently by flicking the tube, and spin down.
Using a thermal cycler, incubate at 20°C for 5 minutes and 65°C for 5 minutes.
Resuspend the AMPure XP beads by vortexing.
Transfer the DNA sample to a clean 1.5 ml Eppendorf DNA LoBind tube.
Add 60 µl of resuspended AMPure XP beads to the end-prep reaction and mix by flicking the tube.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Prepare 500 μl of fresh 80% ethanol in nuclease-free water.
Spin down the sample and pellet on a magnet until supernatant is clear and colourless. Keep the tube on the magnet, and pipette off the supernatant.
Keep the tube on the magnet and wash the beads with 200 µl of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 25 µl nuclease-free water. Spin down and incubate for 2 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Remove and retain 25 µl of eluate into a clean 1.5 ml Eppendorf DNA LoBind tube.
Quantify 1 µl of end-prepped DNA using a Qubit fluorometer - recovery aim >700 ng.
Take forward the repaired and end-prepped DNA into the native barcode ligation step. However, at this point it is also possible to store the sample at 4°C overnight.
5. Native barcode ligation
Material
- Native Barcoding Expansion 1-12 (Q-EXP-NBD104) and 13-24 (Q-EXP-NBD114) if multiplexing more than 12 samples
Consumibles
- Tubos Eppendorf DNA LoBind de 1,5 ml
- Agua sin nucleasas (p. ej., ThermoFisher AM9937)
- NEB Blunt/TA Ligase Master Mix (NEB, M0367)
- Etanol al 80 % recién preparado con agua sin nucleasas
Instrumental
- Gradilla magnética, adecuada para tubos Eppendorf de 1,5 ml
- Mezclador Hula (mezclador giratorio suave)
- Mezclador vórtex
- Cubeta con hielo
- Microcentrífuga
- Pipeta y puntas P1000
- Pipeta y puntas P100
- Pipeta y puntas P10
Equipo opcional
- Fluorímetro Qubit (o equivalente)
Thaw the native barcodes at room temperature. Use one barcode per sample. Individually mix the barcodes by pipetting, spin down, and place them on ice.
Select a unique barcode for every sample to be run together on the same flow cell, from the provided 24 barcodes. Up to 24 samples can be barcoded and combined in one experiment.
Dilute 500 ng of each end-prepped sample to be barcoded to 22.5 µl in nuclease-free water.
Add the reagents in the order given below, mixing by flicking the tube between each sequential addition:
| Reagent | Volume |
|---|---|
| 500 ng end-prepped DNA | 22.5 µl |
| Native Barcode | 2.5 µl |
| Blunt/TA Ligase Master Mix | 25 µl |
| Total | 50 µl |
Mix well by pipetting using wide-bore pipette tips. Alternatively, if you are concerned about preserving the integrity of very long DNA fragments, mix gently by flicking the tube, and spin down.
Incubate the reaction for 10 minutes at room temperature.
Resuspend the AMPure XP beads by vortexing.
Add 50 µl of resuspended AMPure XP beads to the reaction and mix by pipetting.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Prepare 500 μl of fresh 80% ethanol in nuclease-free water.
Spin down the sample and pellet on a magnet. Keep the tube on the magnet, and pipette off the supernatant when clear and colourless.
Keep the tube on the magnet and wash the beads with 200 µl of freshly prepared 80% ethanol without disturbing the pellet. Remove the ethanol using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual ethanol. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 26 µl nuclease-free water. Incubate for 2 minutes at room temperature.
Pellet the beads on a magnet until the eluate is clear and colourless.
Remove and retain 26 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads
Quantify 1 µl of eluted sample using a Qubit fluorometer.
Please first refer to the ligation step below to ensure that the library is diluted to the correct volume.
Pool equimolar amounts of each barcoded sample into a 1.5 ml Eppendorf DNA LoBind tube, ensuring that sufficient sample is combined to produce a pooled sample of 700 ng total.
Quantify 1 µl of pooled and barcoded DNA using a Qubit fluorometer.
Dilute 700 ng pooled sample to 65 µl in nuclease-free water.
If 700 ng of pooled sample exceeds 65 µl in volume, perform an AMPure clean-up with 2.5x Agencourt AMPure XP beads to pooled sample volume, eluting in 65 µl of nuclease-free water.
Fragment size and adapter ligation
The amount of adapter has been optimised for fragment sizes greater or equal to 8 kb. If the fragments are generally smaller than 3 kb, adjustments should be made to use 0.1–0.2 pmoles of DNA in the adapter ligation step.
6. Adapter ligation and clean-up
Material
- Long Fragment Buffer (LFB)
- Short Fragment Buffer (SFB)
- Elution Buffer (EB) from the Ligation Sequencing Kit
- Adapter Mix II (AMII)
Instrumental
- Microcentrífuga
- Gradilla magnética
- Mezclador vórtex
- Mezclador Hula (mezclador giratorio suave)
Equipo opcional
- Fluorímetro Qubit (o equivalente)
Depending on the wash buffer (LFB or SFB) used, the clean-up step after adapter ligation is designed to either enrich for DNA fragments of >3 kb, or purify all fragments equally.
- To enrich for DNA fragments of 3 kb or longer, use Long Fragment Buffer (LFB)
- To retain DNA fragments of all sizes, use Short Fragment Buffer (SFB)
Thaw the Elution Buffer (EB) and NEBNext Quick Ligation Reaction Buffer (5x) at room temperature, mix by vortexing, spin down and place on ice. Check the contents of each tube are clear of any precipitate.
Spin down the T4 Ligase and the Adapter Mix II (AMII), and place on ice.
To enrich for DNA fragments of 3 kb or longer, thaw one tube of Long Fragment Buffer (LFB) at room temperature, mix by vortexing, spin down and place on ice.
To retain DNA fragments of all sizes, thaw one tube of Short Fragment Buffer (SFB) at room temperature, mix by vortexing, spin down and place on ice.
Taking the pooled and barcoded DNA, perform adapter ligation as follows, mixing by flicking the tube between each sequential addition.
| Reagent | Volume |
|---|---|
| 700 ng pooled barcoded sample | 65 µl |
| Adapter Mix II (AMII) | 5 µl |
| NEBNext Quick Ligation Reaction Buffer (5X) | 20 µl |
| Quick T4 DNA Ligase | 10 µl |
| Total | 100 µl |
Ensure the components are thoroughly mixed by pipetting, and spin down.
Incubate the reaction for 10 minutes at room temperature.
Resuspend the AMPure XP beads by vortexing.
Add 40 µl of resuspended AMPure XP beads to the reaction and mix by pipetting.
Incubate on a Hula mixer (rotator mixer) for 5 minutes at room temperature.
Place on a magnetic rack, allow beads to pellet and pipette off supernatant.
Wash the beads by adding either 250 μl Long Fragment Buffer (LFB) or 250 μl Short Fragment Buffer (SFB). Flick the beads to resuspend, spin down, then return the tube to the magnetic rack and allow the beads to pellet. Remove the supernatant using a pipette and discard.
Repeat the previous step.
Spin down and place the tube back on the magnet. Pipette off any residual supernatant. Allow to dry for ~30 seconds, but do not dry the pellet to the point of cracking.
Remove the tube from the magnetic rack and resuspend the pellet in 15 µl Elution Buffer (EB). Spin down and incubate for 10 minutes at room temperature. For high molecular weight DNA, incubating at 37°C can improve the recovery of long fragments.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Remove and retain 15 µl of eluate containing the DNA library into a clean 1.5 ml Eppendorf DNA LoBind tube.
Dispose of the pelleted beads
Quantify 1 µl of adapter ligated and barcoded DNA using a Qubit fluorometer - recovery aim ~430 ng.
We recommend loading 5–50 fmol of this final prepared library onto a flow cell. Loading more than 50 fmol of DNA can have a detrimental effect on throughput. Dilute the library in Elution Buffer if required.
The prepared library is used for loading onto the flow cell. Store the library on ice until ready to load.
7. Priming and loading the MinION Flow Cell
Material
- Flush Tether (FLT)
- Flush Buffer (FB)
- Sequencing Buffer (SQB)
- Loading Beads (LB)
Consumibles
- Tubos Eppendorf DNA LoBind de 1,5 ml
- Agua sin nucleasas (p. ej., ThermoFisher AM9937)
Instrumental
- MinION Flow Cell
- Pipeta y puntas P1000
- Pipeta y puntas P100
- Pipeta y puntas P20
- Pipeta y puntas P10
Thaw the Sequencing Buffer (SQB), Loading Beads (LB), Flush Tether (FLT) and one tube of Flush Buffer (FB) at room temperature before mixing the reagents by vortexing, and spin down at room temperature.
To prepare the flow cell priming mix, add 30 µl of thawed and mixed Flush Tether (FLT) directly to the tube of thawed and mixed Flush Buffer (FB), and mix by vortexing at room temperature.
Slide open the GridION lid and insert the flow cell.
Press down firmly on the flow cell to ensure correct thermal and electrical contact.
Slide the flow cell priming port cover clockwise to open the priming port.
Take care when drawing back buffer from the flow cell. Do not remove more than 20-30 µl, and make sure that the array of pores are covered by buffer at all times. Introducing air bubbles into the array can irreversibly damage pores.
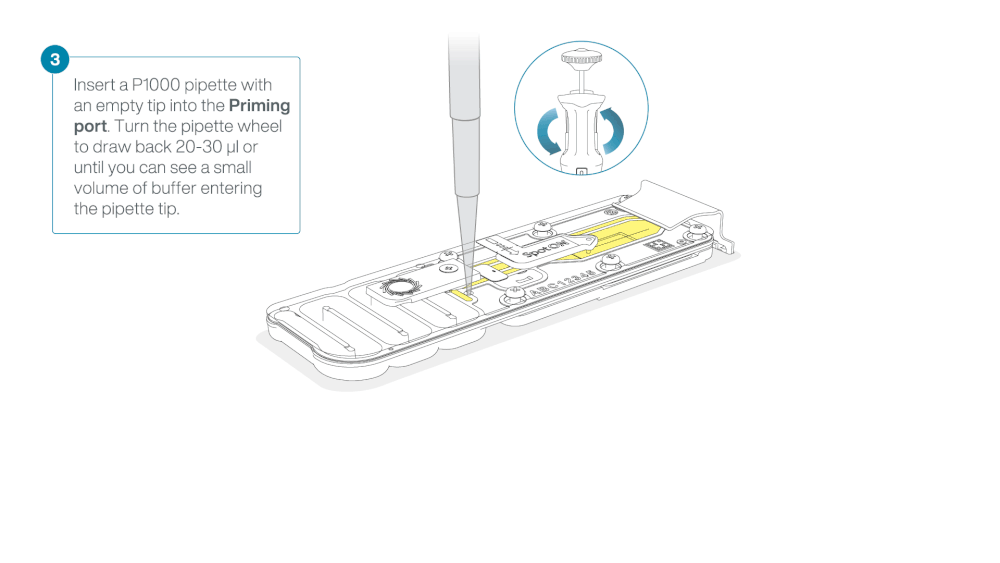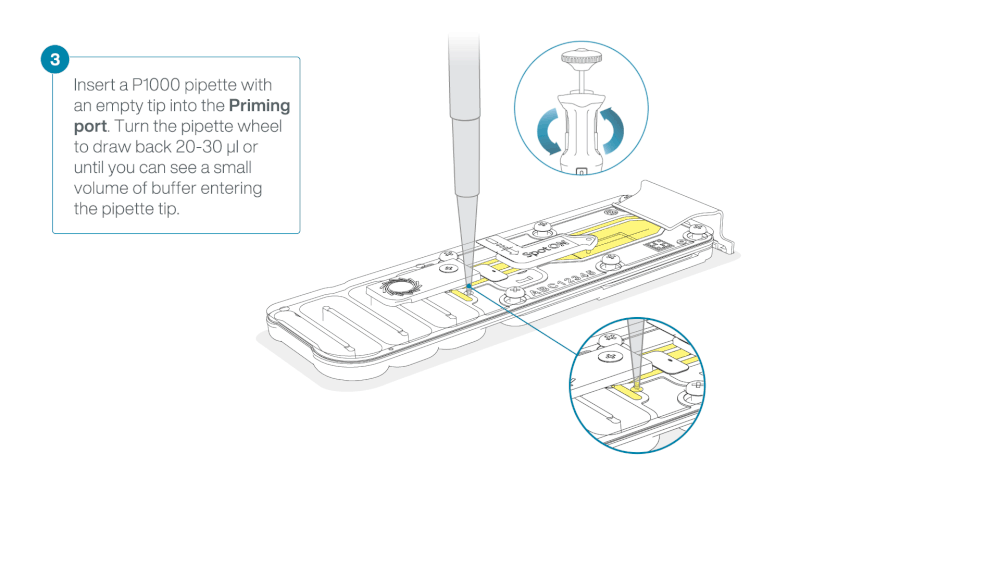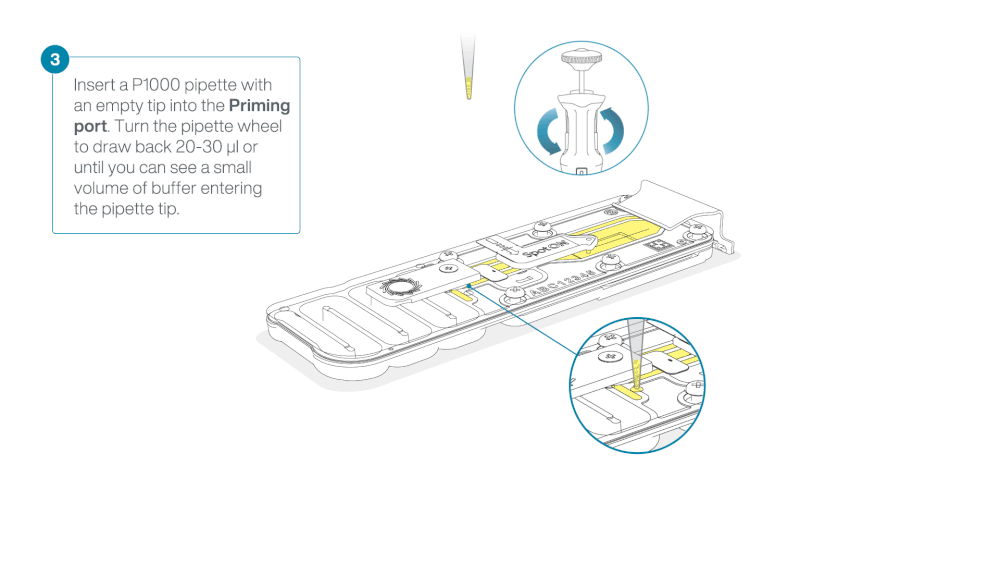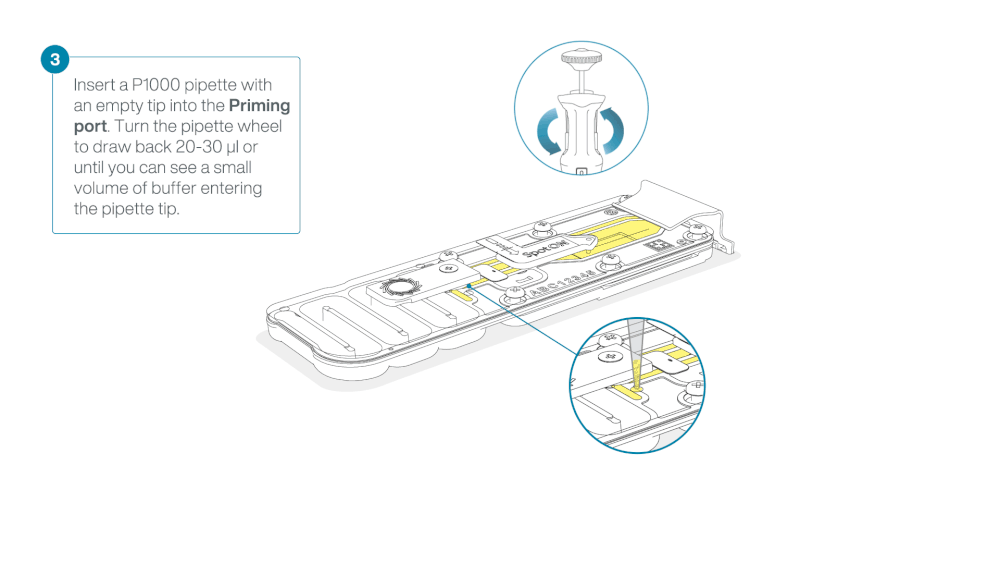
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
- Set a P1000 pipette to 200 µl
- Insert the tip into the priming port
- Turn the wheel until the dial shows 220-230 µl, to draw back 20-30 µl, or until you can see a small volume of buffer entering the pipette tip
Note: Visually check that there is continuous buffer from the priming port across the sensor array.
Load 800 µl of the priming mix into the flow cell via the priming port, avoiding the introduction of air bubbles. Wait for five minutes. During this time, prepare the library for loading by following the steps below.

Thoroughly mix the contents of the Loading Beads (LB) by pipetting.
The Loading Beads (LB) tube contains a suspension of beads. These beads settle very quickly. It is vital that they are mixed immediately before use.
In a new tube, prepare the library for loading as follows:
| Reagent | Volume per flow cell |
|---|---|
| Sequencing Buffer (SQB) | 37.5 µl |
| Loading Beads (LB), mixed immediately before use | 25.5 µl |
| DNA library | 12 µl |
| Total | 75 µl |
Note: Load the library onto the flow cell immediately after adding the Sequencing Buffer (SQB) and Loading Beads (LB) because the fuel in the buffer will start to be consumed by the adapter.
Complete the flow cell priming:
- Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
- Load 200 µl of the priming mix into the flow cell priming port (not the SpotON sample port), avoiding the introduction of air bubbles.

Mix the prepared library gently by pipetting up and down just prior to loading.
Add 75 μl of the prepared library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.

Gently replace the SpotON sample port cover, making sure the bung enters the SpotON port, close the flow cell priming port and close the GridION lid.
8. Data acquisition and basecalling
How to start sequencing
Follow the instructions in the Q-Line sequencing software user guide beginning from the "Set up and run an assay" section.







